Fluorene-containing phenanthrene imidazole derivative and crystal as well as preparation method and application thereof
A technology of phenanthroimidazoles and phenanthroimidazoles, which is applied in the field of organic electroluminescent devices, can solve the problems of short fluorescence lifetime, low luminous intensity, poor thermal stability, etc., and achieve high luminous intensity, high fluorescence quantum yield, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
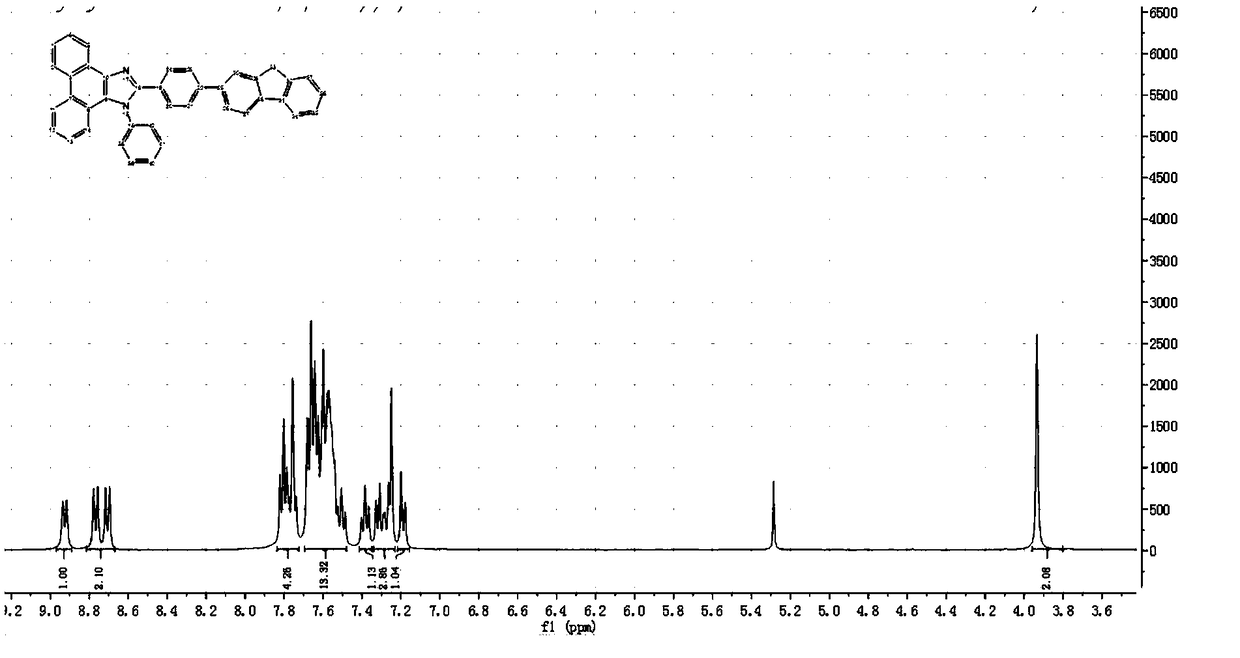
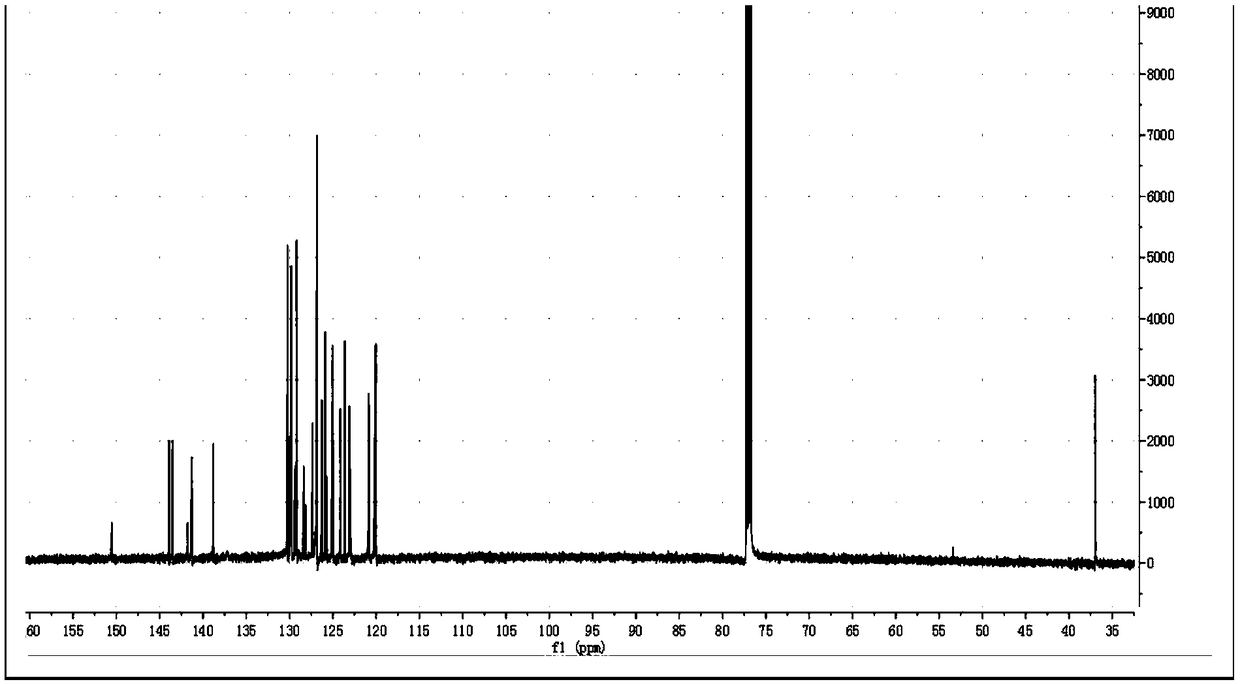
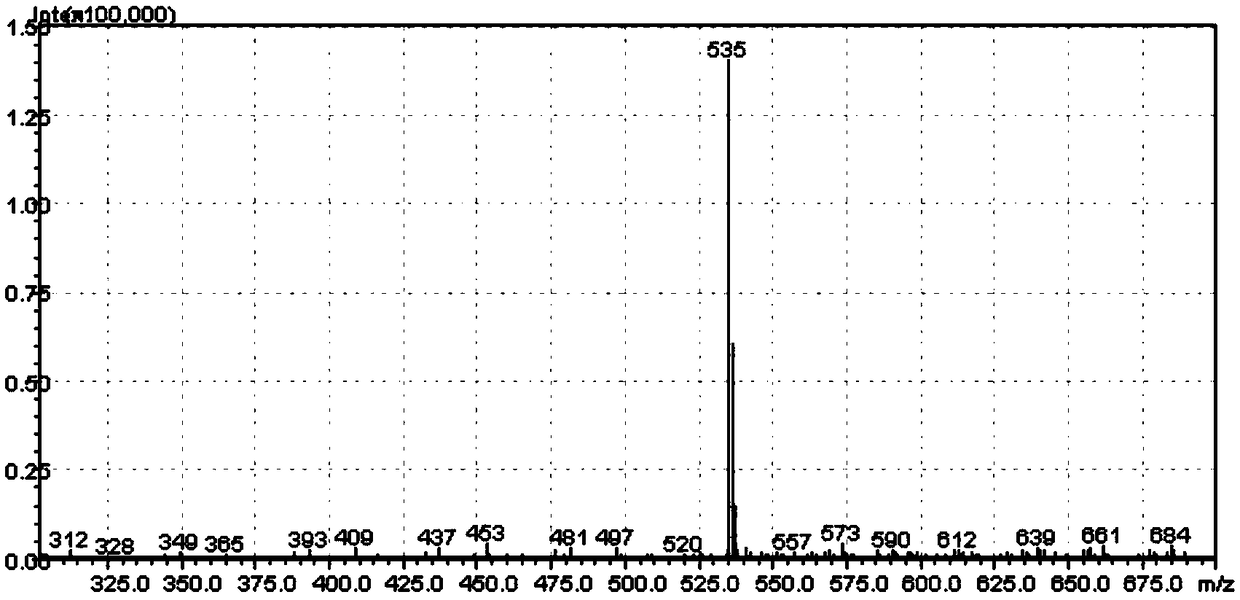
[0048] A fluorene-containing phenanthroimidazole derivative prepared by the following method:
[0049] (1) Preparation of 4-(9H-fluoren-2-yl)benzaldehyde:
[0050] 2.73 g of 2-bromofluorene, 3.34 g of p-formylphenylboronic acid, 0.5 g of tetrakis(triphenylphosphine) palladium, 4.62 g of potassium carbonate, 80 mL of tetrahydrofuran and 20 mL of H 2 O, was added to a 250 mL round bottom flask, the temperature was 70 °C, under the protection of nitrogen, the reaction was heated and stirred under reflux for 12 h. Cool to room temperature and extract with dichloromethane to give a brown solid after desolvation. Using dichloromethane and n-hexane as eluents, 2.52 g of white 4-(9H-fluoren-2-yl)benzaldehyde was obtained by column chromatography with a yield of 84%.
[0051] (2) Preparation of phenanthroimidazole derivatives containing fluorene:
[0052] Add 0.6 g of 4-(9H-fluoren-2-yl)benzaldehyde, 0.32 g of 9,10-phenanthrenequinone, 0.54 g of aniline, 0.39 g of ammonium acetate a...
Embodiment 2
[0056] The difference between this example and Example 1 is that the catalyst in step (1) of this example is 1, 1-bis(diphenylphosphino)ferrocenepalladium dichloride, and the solvent is a mixed solvent of DMF and water .
[0057] Other conditions and operation steps are identical with embodiment 1.
[0058] The yield of 4-(9H-fluoren-2-yl)benzaldehyde obtained in this example was 64%.
Embodiment 3
[0060] The difference between this example and Example 1 is that, in the preparation of the fluorene-containing phenanthroimidazole derivative crystal of this example, the crystal was heated to 300°C.
[0061] Other conditions and operation steps are identical with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Center wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com