A kind of binuclear magnesium metal catalyst and its preparation method and application
A magnesium metal and catalyst technology, applied in the field of dual-nuclear magnesium metal catalyst and its preparation, can solve the problems of low selectivity, insufficient research and the like, and achieve the effects of simple preparation process, easy availability of raw materials and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
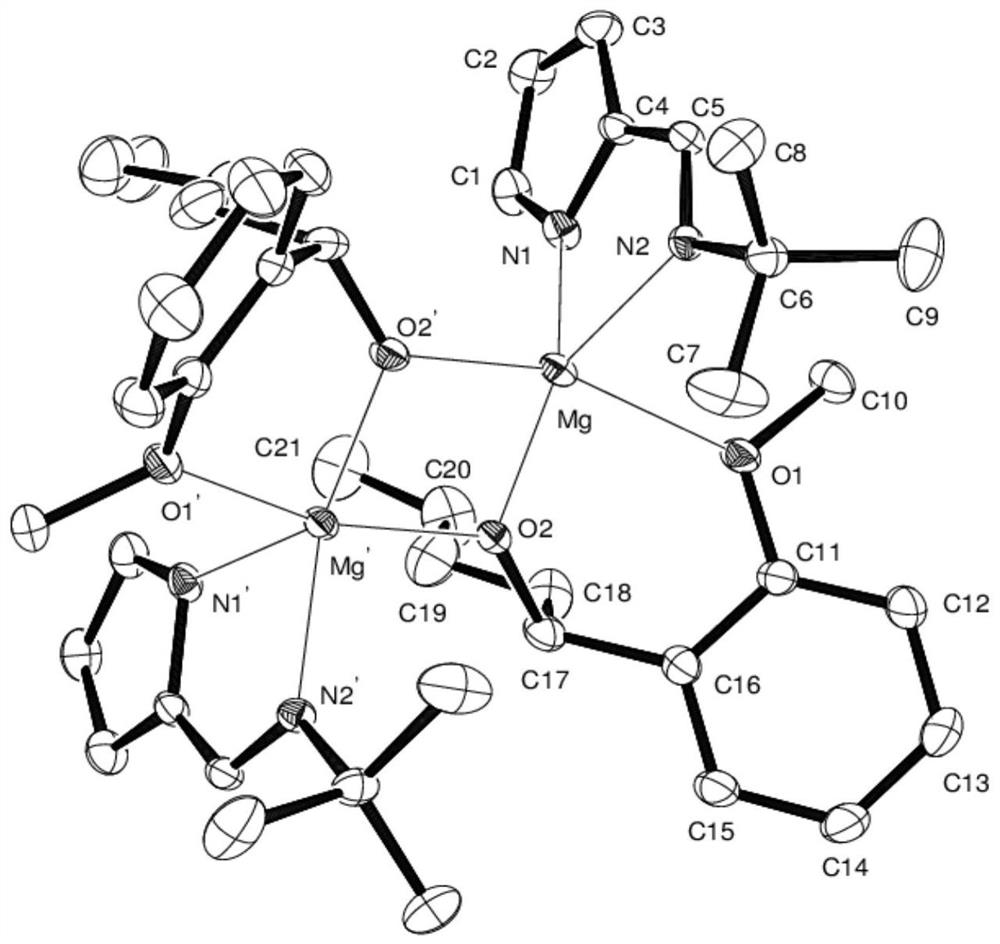
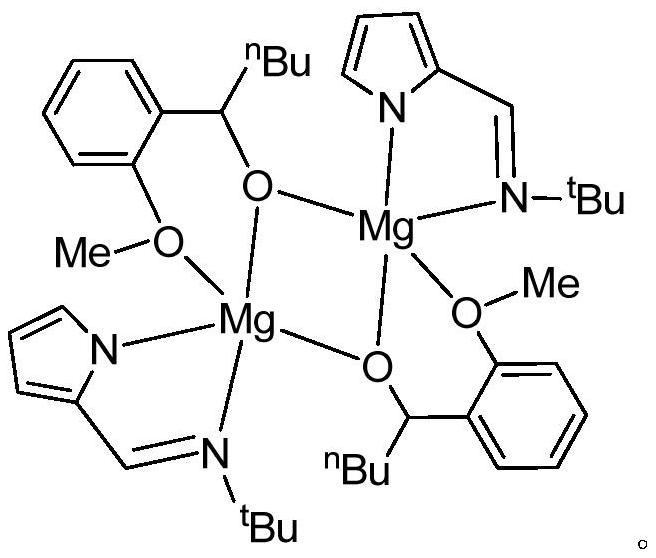
[0016] The preparation of embodiment 1 binuclear magnesium metal catalyst
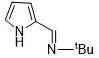
[0017] Under the protection of inert gas and ice-water bath, di-n-butylmagnesium solution (3.00mL, 1.0M) was slowly added dropwise to the n-hexane solution of N-pyrromethene tert-butylamine ligand (0.451g, 3mmol). Complete, return to room temperature naturally, after continuing to stir for 2 hours, the resulting solution is cooled to zero degrees Celsius again with an ice-water bath, and o-methoxybenzaldehyde (0.408g, 3mmol) is added dropwise successively, and slowly returns to room temperature after the addition is completed. Stirring was continued for 7 hours, the reaction solution was concentrated in vacuo, extracted with ether, the extract was filtered and concentrated to a saturated state, left at room temperature for 1-2 days, and colorless transparent crystals were precipitated, which were binuclear magnesium metal catalysts (hereinafter referred to as catalysts), with a yield of 63 %.
[0018]...
Embodiment 2
[0021] (1) The preparation of the catalyst is the same as in Example 1.
[0022] (2) Catalytic benzaldehyde and o-bromobenzaldehyde cross-coupling Tishchenko reaction:
[0023]
[0024] At room temperature, benzaldehyde (0.212g, 2mmol) and o-bromobenzaldehyde (0.370g, 2mmol) were slowly added to the Schlenk reaction flask that catalyst (0.073g, 0.10mmol) was housed along the retort wall, and the temperature was controlled at 30 After stirring the reaction at ℃ for 4 hours, the reaction was terminated with 5 mL of water, extracted three times with ethyl acetate, the extract was concentrated in vacuo, and the crude product was separated by silica gel column chromatography to obtain the target product, a white solid, with a yield of 81%. 1 H NMR (600MHz, CDCl 3 ):δ=5.48(s,2H),7.24(t,1H),7.35(t,1H),7.48(m,2H),7.59(m,3H),8.13(d,J=6.4Hz,2H) ; 13 C (150.9MHz, CDCl 3 ): δ=66.2, 123.5, 127.6, 128.5, 129.7, 129.8, 129.9, 132.9, 133.2, 135.4, 166.2.
Embodiment 3
[0026] (1) The preparation of the catalyst is the same as in Example 1.
[0027] (2) Catalytic benzaldehyde and o-methoxybenzaldehyde cross-coupling Tishchenko reaction:
[0028]
[0029] At room temperature, benzaldehyde (0.212g, 2mmol) and o-methoxybenzaldehyde (0.272g, 2mmol) were slowly added to the Schlenk reaction flask containing the catalyst (0.073g, 0.10mmol) along the retort wall, and the temperature was controlled. After stirring the reaction at 30°C for 4 hours, the reaction was terminated with 5 mL of water, extracted three times with ethyl acetate, the extract was concentrated in vacuo, and the crude product was separated by silica gel column chromatography to obtain the target product, a white solid, with a yield of 79%. M.p.42-44℃. 1 H (600MHz, CDCl 3 ):δ=3.89(s,3H),5.45(s,2H),6.94-6.99(m,2H),7.33-7.36(m,1H),7.44-7.47(m,3H),7.58(t,1H ),8.11(d,2H); 13 C (150.9MHz, CDCl 3 ): δ=55.2, 61.7, 110.0, 120.2, 123.8, 127.9, 128.9, 129.0, 129.4, 129.7, 132.5, 157....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com