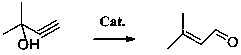3-methylcrotonaldehyde synthesis process
A technology of prenaldehyde and synthesis process, which is applied in the field of prenaldehyde synthesis process, can solve the problems of unreusable reaction time, difficulty in catalyst recovery and mechanical application, low yield of prenaldehyde, etc., and achieves a reduction in yield , easy separation, high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 A kind of synthetic technique of prenaldehyde
[0041] Include the following steps:
[0042] (1) Feeding
[0043] Add 500g of 2-methyl-3-butyn-2-ol (purity 99.7%), 5g of molybdenum trioxide, and 2.0g of triethylamine into a 1L autoclave with magnetic stirring and temperature controller.
[0044] (2) Synthesis reaction
[0045] Replaced 3 times with nitrogen, controlled the reaction temperature at 180°C, stirred at 500rpm, continuously sampled, and analyzed by gas chromatography, the content of raw material 2-methyl-3-butyn-2-ol dropped from 99.7% to 56% and no longer Change, that is, the conversion rate remains unchanged, the reaction is balanced, and the end point is reached, and the reaction lasts for 20 min.
[0046] (3) Separation of catalysts
[0047] Then cool down to room temperature with water, press out the reaction liquid, filter and separate the catalyst, and the catalyst can be recycled.
[0048] (4) Distillation
[0049] After the reactio...
Embodiment 2-9
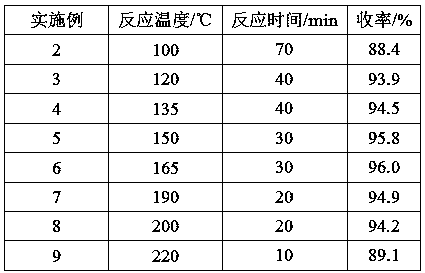
[0055] Adopt the method for embodiment 1, change temperature of reaction, its result is as shown in table 2.
[0056] Table 2 Effect of temperature on reaction results.
[0057]
Embodiment 10-11
[0059] Adopt the method of embodiment 1, change auxiliary agent type, its result is shown in table 3.
[0060] Table 3 Effect of additive types on reaction results.
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com