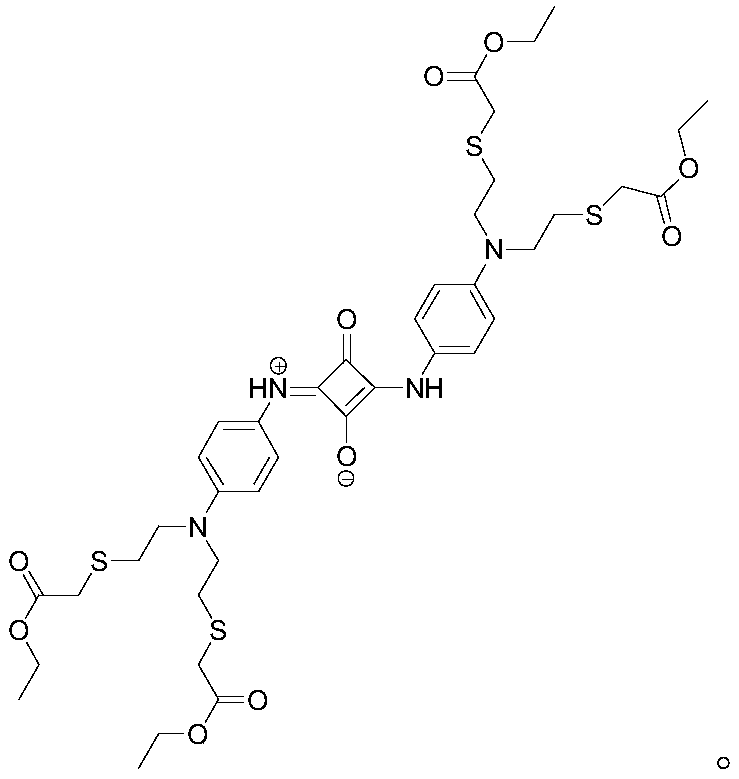
Probe based on 1-and-3-symmetric squarine recognizing silver ions as well as preparation method and application thereof
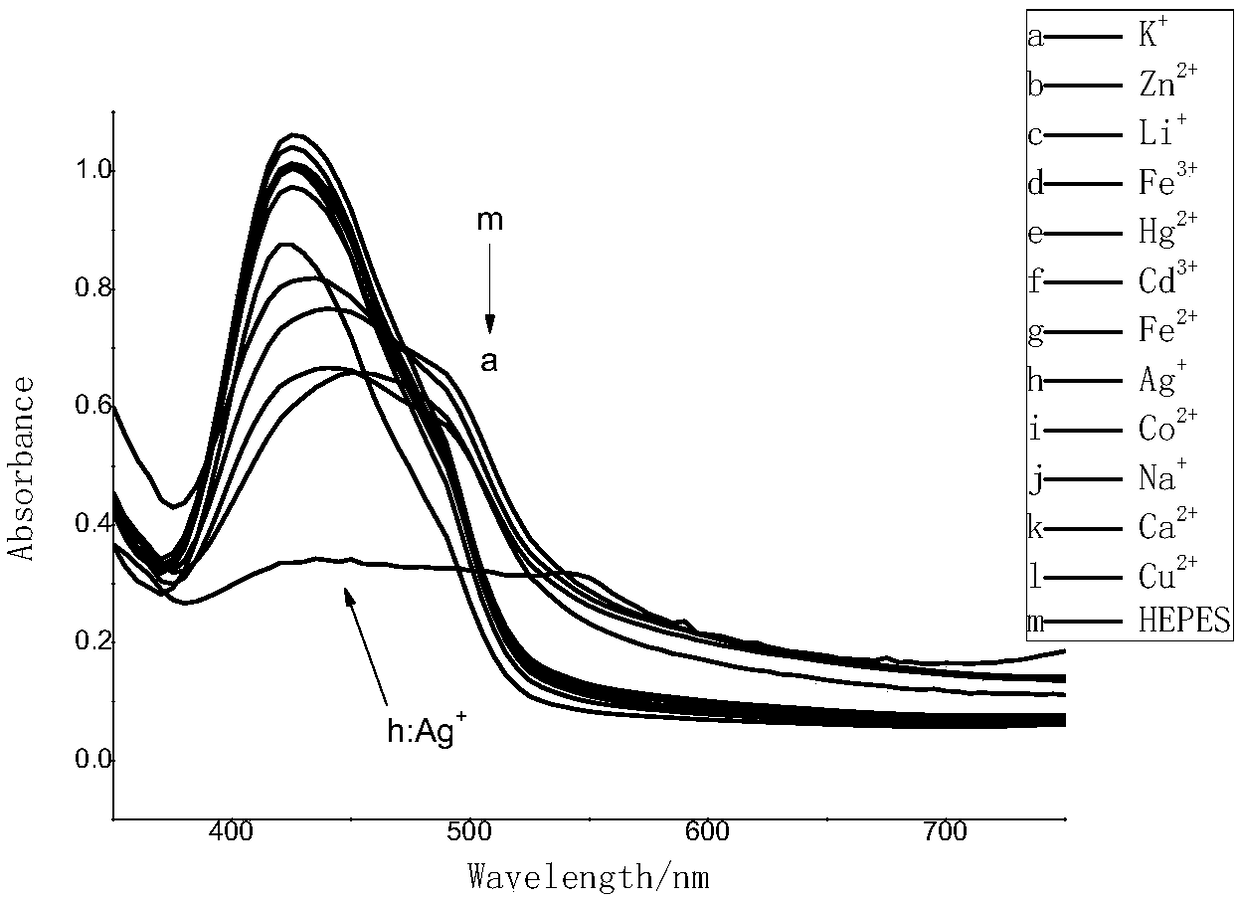
A silver ion and probe technology, applied in the field of squaraine probe and its preparation, can solve the problems of high cost and long detection time, and achieve the effects of high sensitivity, high reaction yield and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
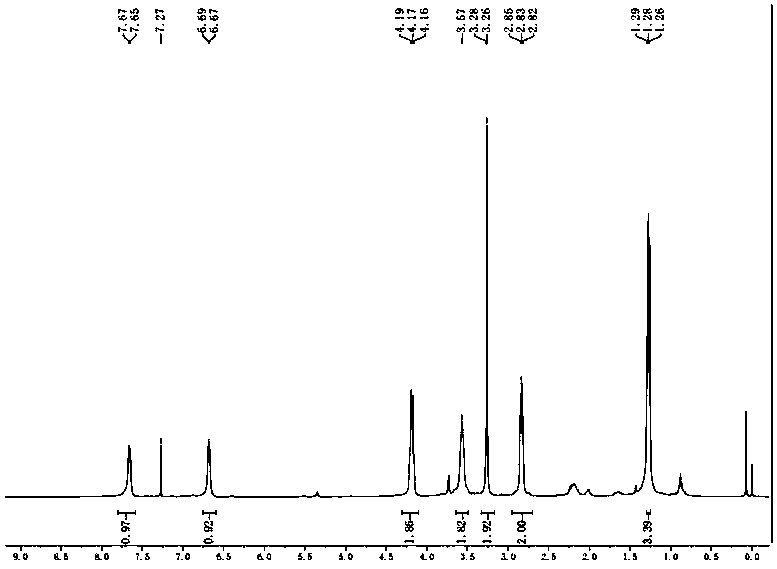
[0027] (1) Fully disperse compound 1 (N-(4-nitrophenyl)diethanolamine, 1.13g, 5mmol) and p-toluenesulfonyl chloride (TsCl) (1.9g, 10mmol) in 5ml of tetrahydrofuran (THF) and 5ml In a mixture of water and reaction at room temperature for 2 h to obtain compound 2 (p-toluenesulfonyl derivative of compound 1), which was separated and purified by column chromatography to obtain 2.0 g with a yield of 74.5%;
[0028] (2) Compound 2 (2g, 3.7mmol) was added dropwise to methyl thioglycolate (0.8g, 7.4mmol) and cesium carbonate (Cs 2 CO 3 , 0.26g, 0.74mmol) of anhydrous N,N-dimethylformamide (DMF), wherein the amount of anhydrous N,N-dimethylformamide is 15ml, and reacted at a reaction temperature of 60°C for 2 days , separated and purified by column chromatography to obtain 1.2 g of compound 3 (ethylsulfanyl-methyl acetate derivative of compound 1), with a yield of 80%;
[0029] (3) Using Pb / C (0.06g, 5%) as a catalyst, hydrazine hydrate (0.45ml, 7.5mmol) as a reducing agent, compound...
Embodiment 2
[0036] (1) Compound 1 (N-(4-nitrophenyl)diethanolamine (3.2g, 14mmol) and p-toluenesulfonyl chloride (TsCl) (5.3g, 28mmol) were dissolved in 10ml of tetrahydrofuran (THF) and 10ml of water The mixture was reacted at room temperature for 4 h to obtain compound 2 (the p-toluenesulfonyl derivative of compound 1) which was separated and purified by column chromatography to obtain 5.4 g with a yield of 71.3%;
[0037] (2) Compound 2 (5.4g, 10mmol) was slowly added dropwise to methyl thioglycolate (2.16g, 20mmol) and cesium carbonate (Cs 2 CO 3 , 1.1g, 2mmol) of anhydrous N,N-dimethylformamide (DMF), wherein the amount of anhydrous N,N-dimethylformamide is 25ml, reflux at 60°C for 3 days, and pass through the column Chromatographic separation and purification yielded 2.62 g of compound 3 (ethylsulfanyl-methyl acetate derivative of compound 1), with a yield of 65.2%;
[0038] (3) Using Pb / C (0.18g, 5%) as a catalyst, hydrazine hydrate (1ml, 8.2mmol) as a reducing agent, compound 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com