A thin-layer chromatography detection method for amino acid impurities in l-2-amino-5-guanidinovaleric acid raw materials
A technique of arginine and thin-layer chromatography, applied in the field of food and drug analysis, can solve the problems of low sensitivity and poor separation effect, and achieve the effect of solving the problem of detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: L-2- amino-5-guanidino-pentanoic acid detection sensitivity of the detection
[0063] 1, the sample solution preparation: Weigh L-2- amino-5-guanidino valeric acid was dissolved 0.1g in 10mL water, diluted with 1mL of the solution was taken with water to 50mL stand. The solution 0.5,1.0,1.5,2.0 and 2.5 mL were collected, placed in a 10mL volumetric flask, add water to the mark, respectively, formulated as 0.1%, 0.2%, 0.3%, 0.4% and 0.5% of L-2- amino - 5-guanidino-pentanoic acid solution.
[0064]2, thin layer chromatography: neutral were prepared of a hard glass tube from step 1 different concentrations of L-2- amino-5-guanidino-pentanoic acid 5 L of solution, spotted on the same TLC plate of silica gel G; then, spotted TLC plate was placed in a chromatography tank, added isopropanol - concentrated ammonia solution developing solvent: rear (73), expand about 10cm at room temperature for 26 ℃ environment of 74% relative humidity and drying out; then the TLC plate...
Embodiment 2
[0066] Example 2: Location-amino-5-guanidino-pentanoic acid homologs impurity L-2-
[0067] 1, was prepared:
[0068] (1) homolog solution: Weigh L-2- amino-succinic acid, L-2,5- diamino pentanoic acid, ( S ) -2-amino-5-ureido pentanoic acid, ( S ) -2-acetylamino-5-amino-valeric acid, ( S ) -2-acetylamino-oxoglutarate 2mg each placed in a different flask, dissolved in water and dilute to 10mL ,, homologs obtained solution concentration were 0.2mg / mL of solution were referred to as A2, the solution B2, a solution of C2, the solution D2, solution E2.
[0069] (2) The system suitability solution: Weigh accurately L-2- amino-5-guanidino valeric acid, and two L-2,6- reference aminocaproic acid, diluted with water and made, respectively, each containing about l mL L- amino-5-guanidino-pentanoic acid solution, and 20 mg L-2,6- diamino-hexanoic acid 0.4mg, denoted solution F2.
[0070] 2, thin layer chromatography: neutral, respectively, of a hard glass tube of each step taking 5μL homo...
Embodiment 3
[0072] Example 3: homologs L-2- amino-succinic acid formulation and a method for determining the concentration of
[0073] 1, was prepared:
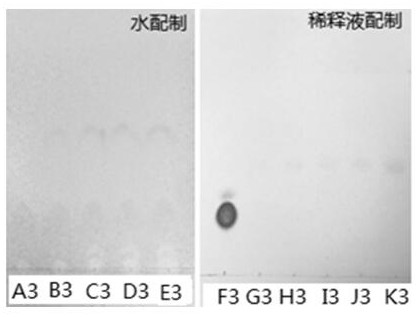
[0074] (1) L-2- amino-water formulation succinic acid solution: Weigh accurately L-2- amino-succinic acid 10mg, is dissolved with dilute phosphoric acid was added after the water volume to 10mL, as a solution; respectively weighed 1mL, 0.8mL , 0.6mL, 0.4mL, 0.2mL solution 1, was placed in different volumetric flask, dilute to volume with water to 10mL, obtained at a concentration of 0.10mg / mL, 0.08mg / mL, 0.06mg / mL, 0.04mg / mL, 0.02 mg / mL of L-2- amino-succinic acid solution, a solution denoted as A3, solution B3, solution C3, the solution D3, a solution of E3.
[0075] (2) Preparation of L-2- amino-succinic acid solution was diluted with: sodium hydroxide solution to adjust the pH to 10.0 to 12.0 for aqueous dilution. Accurately weighed L-2- amino-succinic acid 10mg, is dissolved with the diluent and set the volume to 10mL, as a sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com