Detection method of L-2-amino-5-guanidinovaleric acid enantiomer
A technology for enantiomers and arginyl valeric acid is applied in the field of detection of L-2-amino-5-arginyl valeric acid enantiomers, and can solve problems such as no relevant reports on the analysis and separation method, and the like, To achieve the effect of ensuring food and medicinal safety, good separation, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Detection of enantiomers of three batches of L-2-amino-5-guanidinovaleric acid bulk drug
[0051] Instrument and chromatographic conditions:
[0052] Agilent 1260 high performance liquid chromatography was used, with octadecylsilane bonded silica gel as filler (4.6×250mm, 5μm), column temperature was 25°C; flow rate was 1.0mL / min; detection wavelength was 250nm; injection volume Take 1.36 grams of potassium dihydrogen phosphate, add water to dissolve and dilute to 1000mL, use 20% phosphoric acid to adjust the pH value to 2.8 as mobile phase A, use methanol as mobile phase B, and perform elution in the following table, and the ratio is shown in the table 1 shows:
[0053] Table 1
[0054]
[0055]
[0056] Precisely measure 100 μl each of the test solution and the enantiomer reference solution, and add an equal volume of derivatization test solution 1 (take 2,3,4,6-tetra-O-acetyl-β-D-pyridine Add an appropriate amount of glucopyranose isothiocyanate (...
Embodiment 2
[0067] Example 2: Enantiomeric Orientation Test
[0068] Instrument and chromatographic conditions are the same as in Example 1.
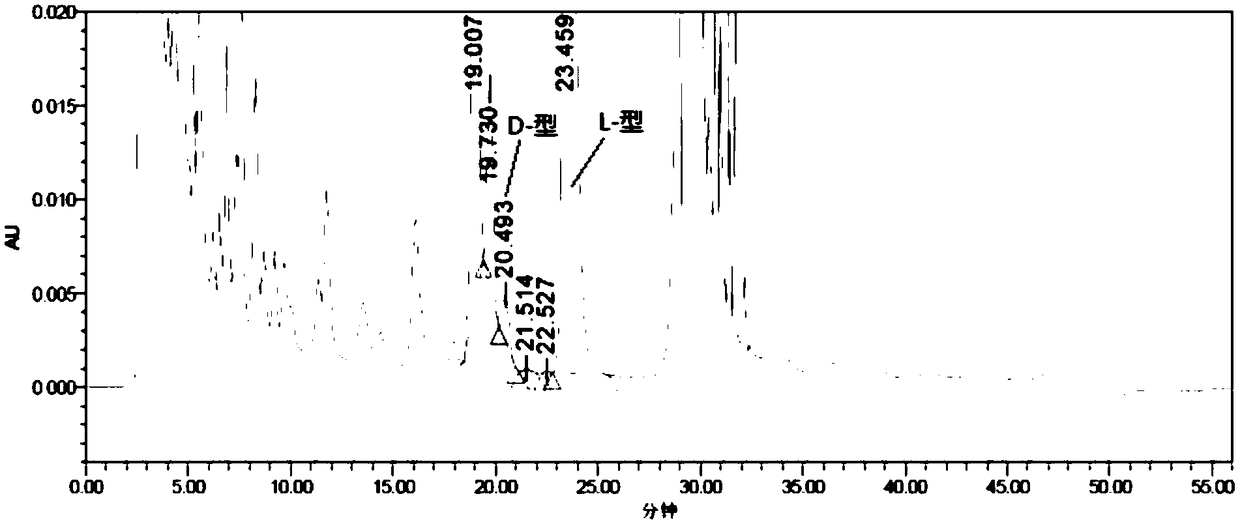
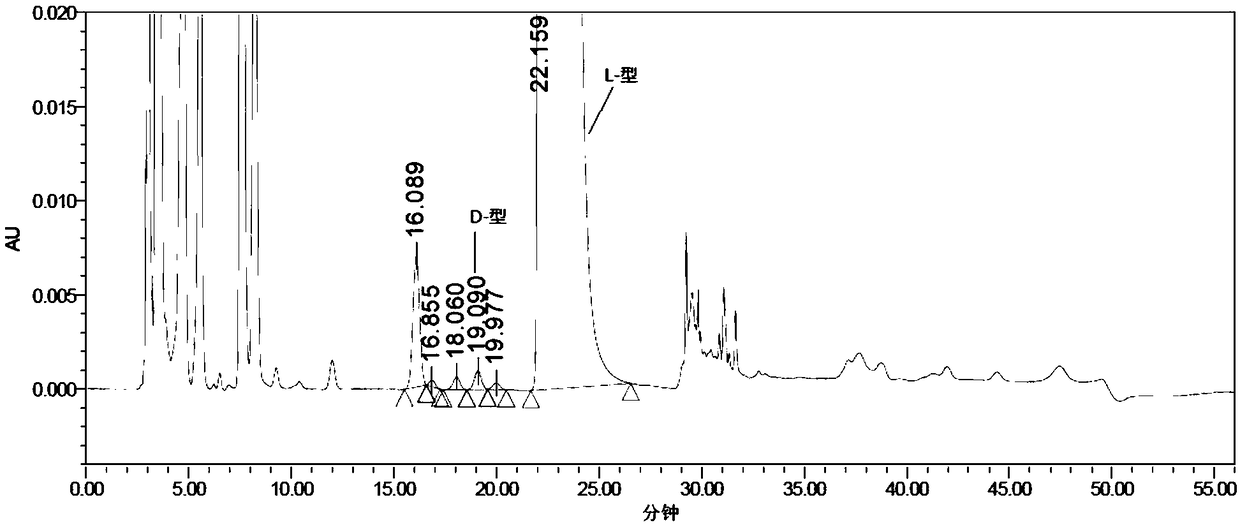
[0069] Experimental procedure: Weigh an appropriate amount of L-2-amino-5-guanidinovaleric acid and its enantiomer reference substance respectively, dissolve them in diluent and quantitatively dilute them to make a solution containing about 0.5mg per 1mL as a positioning solution The preparation method of the mixed solution is the same as the system suitability solution of Example 1, and then derivatized before the column, and each derivatized solution is injected into the liquid chromatograph respectively, and the chromatogram is recorded. The separation results of the derivative peaks of L-2-amino-5-guanidinovaleric acid enantiomers and adjacent peaks are shown in Table 4, and the chromatograms are shown in Figure 5 :
[0070] Table 4
[0071]
[0072] The results showed that: L-2-amino-5-guanidinovaleric acid enantiomeric derivative peaks...
Embodiment 3
[0073] Embodiment 3: Specific destruction test
[0074] Instrument and chromatographic conditions are the same as in Example 1.
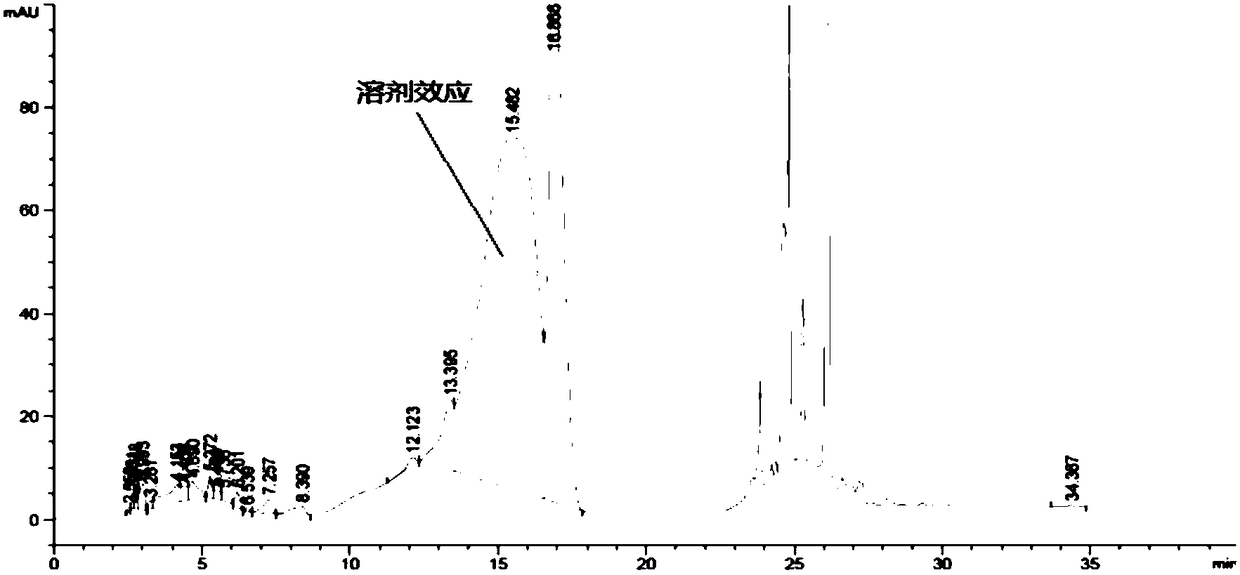
[0075] Experimental procedure: Accurately weigh an appropriate amount of L-2-amino-5-guanidinovaleric acid, and conduct a forced destruction test on it under the conditions of 0.5mol / L hydrochloric acid solution, high temperature of 180°C and strong light of 4500Lx, and the preparation method of each destroyed sample See Table 5, chromatogram see Figure 6 ~ Figure 9 :
[0076] Prepare each specific destruction sample solution in the following table:
[0077] table 5
[0078]
[0079] Take the above-mentioned derivatization solutions and inject samples respectively and record the chromatograms. The destruction results are shown in Table 6:
[0080] Table 6
[0081]
[0082]
[0083] The results showed that no D-2-amino-5-guanidine valeric acid was detected in the samples destroyed by strong acid, high temperature and strong light, indi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com