Rapid separation type soluble microneedle and preparation method thereof
A soluble and separable technology, used in microneedles, medical preparations with inactive ingredients, needles, etc., can solve the problems of prolonging the dissolution rate of the intermediate layer, limited amount of tissue fluid exudation, inaccurate drug dosage, etc., achieving no waste. The effect of secondary injury, efficient drug delivery efficiency, and accelerated dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
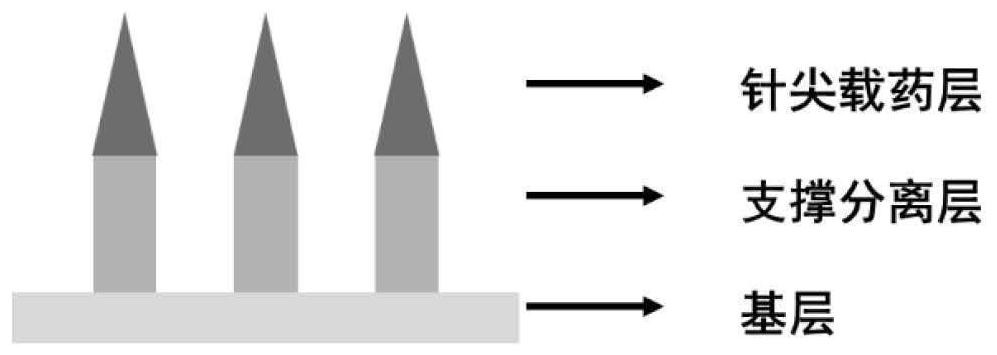
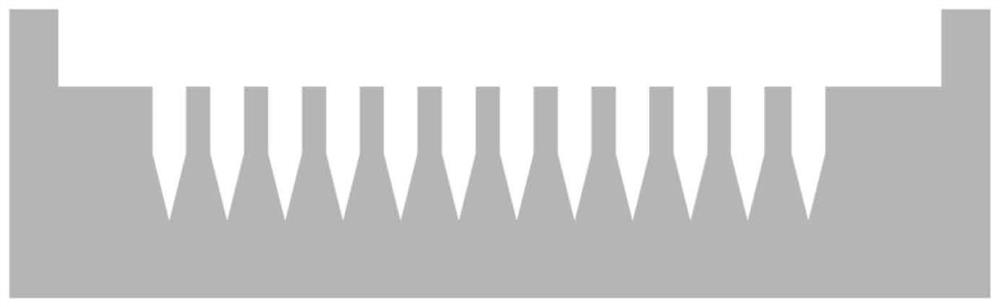
[0051] This embodiment provides a quick-separation soluble microneedle whose support separation layer is hyaluronic acid and sucrose, including a base layer and a needle body, wherein the needle body is composed of a needle tip drug-loaded layer and a support separation layer, and the support separation layer is located at the needle tip Between the drug-loaded layer and the base layer, it is used to connect the needle tip drug-loaded layer and the base layer. The schematic diagram of the structure is as follows figure 1 shown. In this embodiment, the total length of the needle body is 1000 μm; the shape of the drug-loaded layer at the needle tip is a quadrangular pyramid with a height of 450 μm; the shape of the support separation layer is a quadrangular prism with a height of 550 μm; the lengths of the bottom edges are both 250 μm.
[0052] The preparation method comprises the following steps:
[0053] 1) Preparation of needle tip drug-loaded layer solution
[0054] Weigh ...
Embodiment 2
[0064] This embodiment provides a quick-separation soluble microneedle whose supporting separation layer is polyvinylpyrrolidine K17PF and sucrose and a preparation method thereof.
[0065] The microneedle includes a base layer and a needle body, wherein the needle body is composed of a needle point drug-loaded layer and a support separation layer, and the support separation layer is located between the needle point drug-loaded layer and the base layer, and is used for connecting the needle point drug-loaded layer and the base layer. In this embodiment, the total length of the needle body is 1050 μm; the shape of the drug-loaded layer at the needle tip is a square pyramid with a height of 450 μm; the shape of the supporting separation layer is a square prism with a height of 600 μm; the lengths of the bottom edges are all 300 μm.
[0066] The preparation method comprises the following steps:
[0067] 1) Preparation of needle tip drug-loaded layer solution
[0068] Polyvinyl a...
Embodiment 3
[0094] For the soluble microneedles prepared in Example 1 and Comparative Example 1, the separation test of the drug-loaded layer at the needle tip and the base layer was carried out. The specific method is as follows:
[0095]Take an appropriate amount of gelatin and add it to ultrapure water to fully swell for 30 minutes, then transfer it to a 65°C water bath, heat and stir until it is completely dissolved so that its mass fraction is 20%. Pour the dissolved gelatin solution into a petri dish of a suitable size while it is hot, so that the height of the gelatin solution in the petri dish is about 1 cm. When the temperature of the gelatin solution drops to room temperature, a gel with a certain hardness and immobile is formed to start the separation of the microneedle patch. sex test. Cut the gelatin gel into an appropriate size and place it under a microscope, press the microneedles cut into a single row onto the surface of the gelatin gel, observe and record the dissolution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com