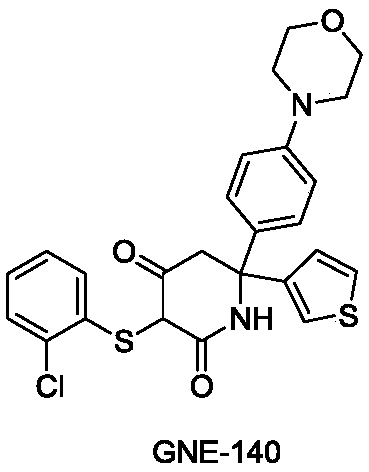
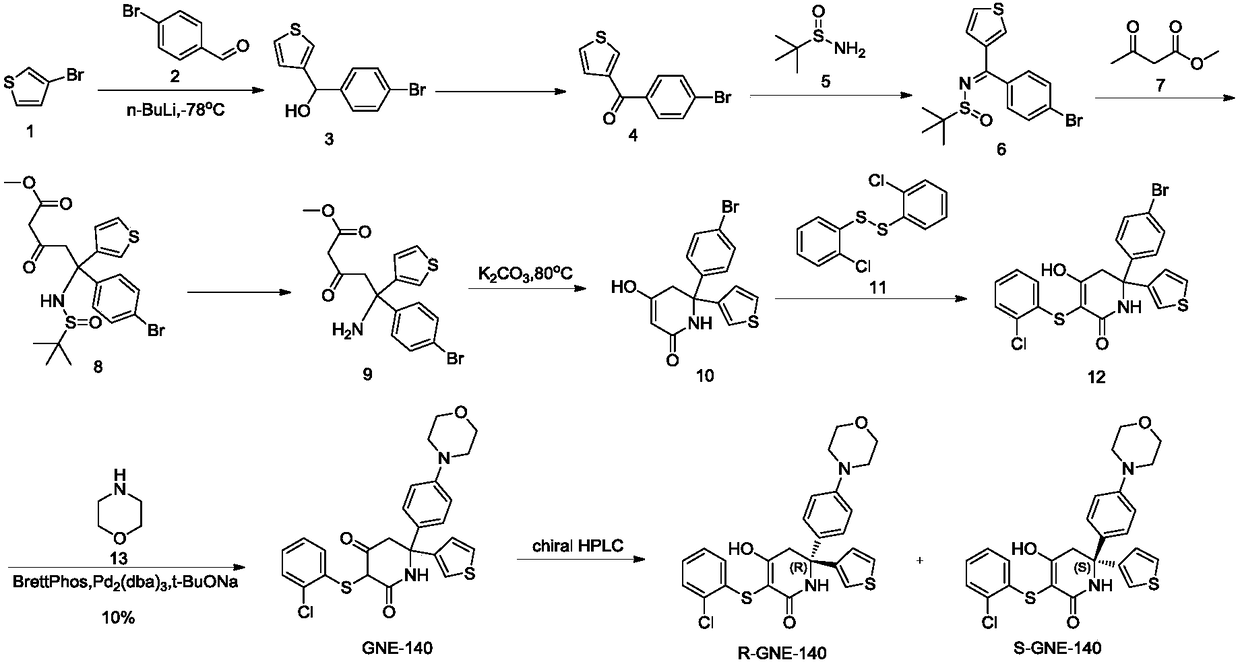
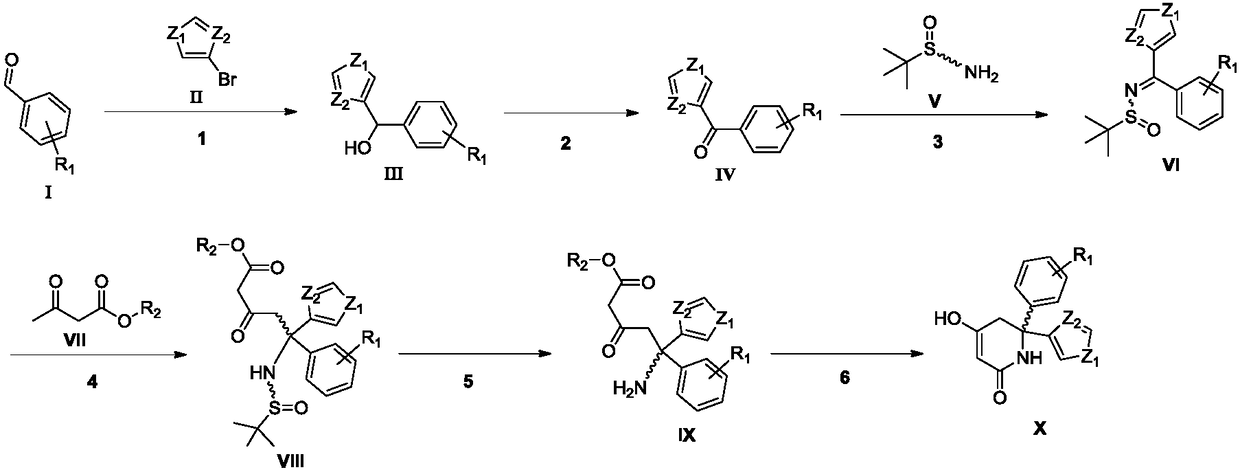
Synthesizing method of key intermediate for synthesizing lactate dehydrogenase A (LDHA) inhibitor
A lactate dehydrogenase and inhibitor technology, which is applied in the field of synthesis of 6,6-aromatic ring-substituted-2,4-piperidinedione, can solve the problem of being unsuitable for industrial scale-up production, low atom economy and difficult by-products. Separation and other problems, to achieve the effect of atom economy, avoid low temperature reaction, good methodological significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Step (1) Preparation of Compound 2
[0048]
[0049] Compound 3-bromothiophene (228g, 1.4mol) was dissolved in anhydrous tetrahydrofuran (2.3L), and isopropylmagnesium chloride (1L, 1.4mol, 1.4M tetrahydrofuran solution) was added at room temperature, and compound 1 was added after stirring for 2 hours ( 267g, 1.4mol), continue stirring at room temperature. After TLC monitors that the reaction is complete, slowly add saturated ammonium chloride solution (2L) to the reaction solution, and leave it to stand after stirring at room temperature for 20 minutes, separate the organic phase, add ethyl acetate (1L×3) to the aqueous layer for extraction, and combine The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated, the crude product was dispersed in ethyl acetate / petroleum ether mixed solution (200mL / 300mL) and stirred overnight, filtered and dried to obtain yellow solid compound 2 (317g, harvested Rate: 82%, 1 ...
Embodiment 2
[0069] Step (1) Preparation of Compound 2
[0070]
[0071] The compound 3-bromothiophene (366g, 2.2mol) was dissolved in anhydrous tetrahydrofuran (2.9L), and isopropylmagnesium chloride-lithium chloride (1.7L, 2.2mol, 1.3M tetrahydrofuran solution) was added at room temperature, and stirred for 1.5 hours Then compound 1 (210 g, 1.1 mol) was added, and stirring was continued at room temperature. After the completion of the reaction monitored by TLC, the compound 2 (282 g, yield: 93%) was purified as a yellow solid according to the post-treatment method of step (1) of Example 1.
[0072] Step (2) Preparation of Compound 3
[0073]
[0074] Dissolve compound 2 (83g, 0.3mol) in dichloromethane (1.2L), add manganese dioxide (35g, 0.4mol) and stir at room temperature. After TLC monitoring shows that the reaction is complete, according to Example 1 step (2) Purified by the post-processing method to obtain yellow solid compound 3 (68 g, yield: 83%).
[0075] Step (3) Prepar...
Embodiment 3
[0090] Step (1) Preparation of Compound 2
[0091]
[0092] The compound 3-bromothiophene (196g, 1.2mol) was dissolved in anhydrous tetrahydrofuran (2L), and isopropylmagnesium chloride-lithium chloride (923mL, 1.2mol, 1.3M tetrahydrofuran solution) was added at room temperature, stirred for 1.5 hours and then added Compound 1 (153 g, 800 mmol), continued stirring at room temperature. After the completion of the reaction monitored by TLC, the compound 2 (198 g, yield: 90%) was purified as a yellow solid according to the post-treatment method of step (1) of Example 1.
[0093] Step (2) Preparation of Compound 3
[0094]
[0095] Dissolve compound 2 (110g, 0.4mol) in dichloromethane (1.5L), add PCC (112g, 0.5mol) and stir at room temperature. After TLC monitoring shows that the reaction is complete, according to the step (2) of Example 1, The work-up method was purified to obtain compound 3 (85 g, yield: 78%) as a yellow solid.
[0096] Step (3) Preparation of compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com