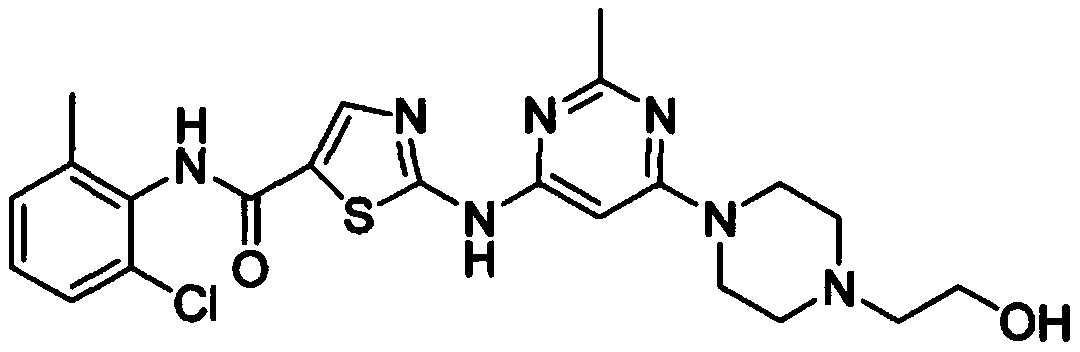
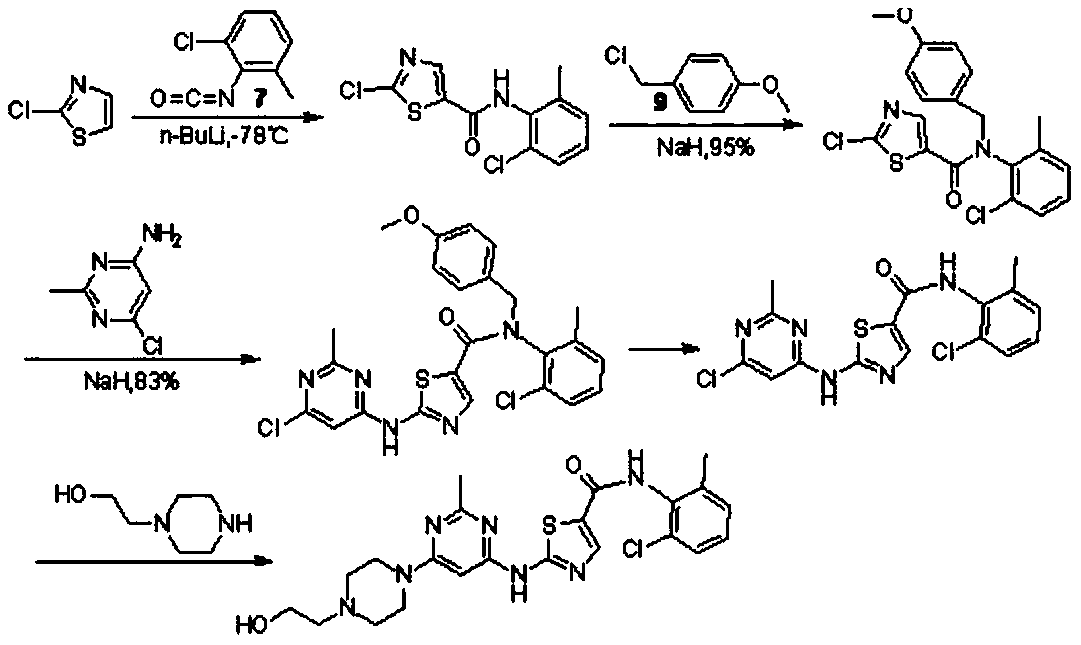
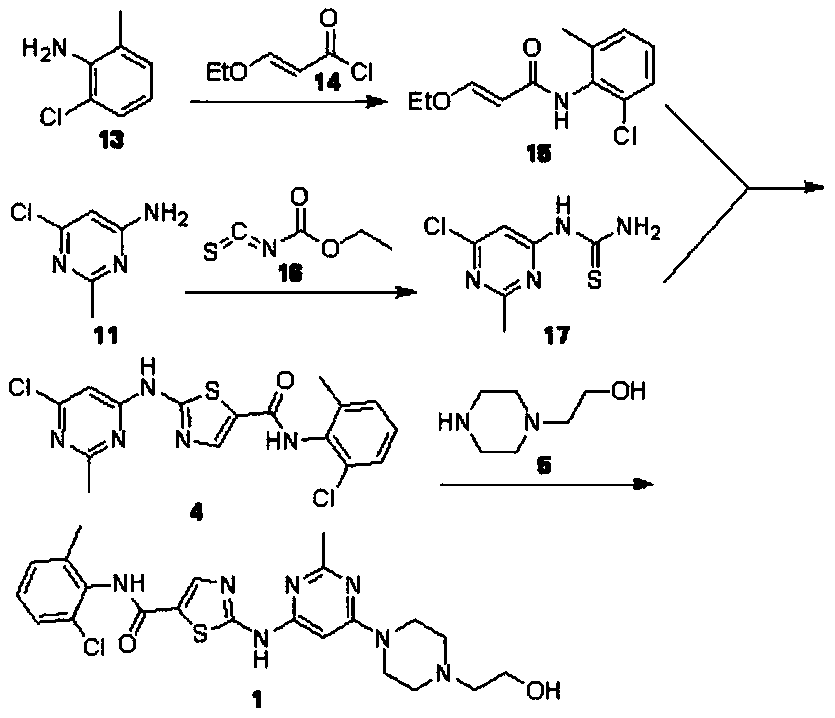
A kind of preparation technology of dasatinib
A technology of dasatinib and its preparation process, which is applied in the field of drug synthesis and can solve the problems of easy polymerization, increased cost, and reduced yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
[0042] Dissolve 30mmol of ethyl 3-oxopropionate and 36mmol of sodium methoxide in 80mL of tetrahydrofuran, stir at room temperature for 10min, add 27mmol of 2-chloro-6-methylaniline, heat up and reflux for 45min, after the reaction, add 60mL of 78mmol Copper bromide in tetrahydrofuran, heated to reflux for 1h, filtered while hot, kept the filtrate, added 39mmol thiourea, 2.4mmol (NH 4 ) 3 [PMo 12 o 40 ], stirred and reacted at 20-25°C for 15 minutes, and monitored the reaction by TLC (following the reaction until the disappearance of the raw materials). After drying, 6.58 g of 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide was obtained, with a yield of 90.94% and a purity of 99.88%.
Embodiment 2
[0043] Example 2: Preparation of 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
[0044] Dissolve 30mmol of ethyl 3-oxopropionate and 39mmol of sodium methoxide in 80mL of tetrahydrofuran, stir at room temperature for 10min, add 27mmol of 2-chloro-6-methylaniline, heat up and reflux for 45min, after the reaction is over, add 60mL of 84mmol Copper bromide in tetrahydrofuran, heat to reflux for 1h, filter while hot, keep the filtrate, add 42mmol thiourea, 2.4mmolH to the filtrate 18 N 3 o 43 PW 12 , stirred and reacted at 20-25°C for 15 minutes, TLC monitored the reaction (followed the reaction until the disappearance of the raw materials), after the reaction, filtered the catalyst, evaporated the filtrate to remove the solvent under reduced pressure, added 50 mL of ether, stirred and crystallized for 20 minutes, suction filtered and dried Finally, 6.59 g of 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide was obtained, with a yield of 91.07% and a purity ...
Embodiment 3
[0045] Example 3: Preparation of 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
[0046] Dissolve 30mmol of ethyl 3-oxopropionate and 30mmol of sodium methoxide in 80mL of tetrahydrofuran, stir at room temperature for 10min, add 24mmol of 2-chloro-6-methylaniline, heat up and reflux for 45min, after the reaction, add 60mL of 60mmol Copper bromide in tetrahydrofuran, heat to reflux for 1h, filter while hot, keep the filtrate, add 30mmol thiourea, 1.5mmol (NH 4 ) 3 [PMo 12 o 40 ], stirred and reacted at 20-25°C for 15 minutes, and monitored the reaction by TLC (following the reaction until the disappearance of the raw materials). After drying, 5.50 g of 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide was obtained, with a yield of 85.31% and a purity of 99.69%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com