Huperzine A sustained-release pellets and preparation method thereof
A technology of huperzine A and sustained-release pellets is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve the problem of reducing single dose, taking less medicine or taking more medicine , memory function decline and other problems, to achieve good drug content uniformity, good compatibility and good formability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
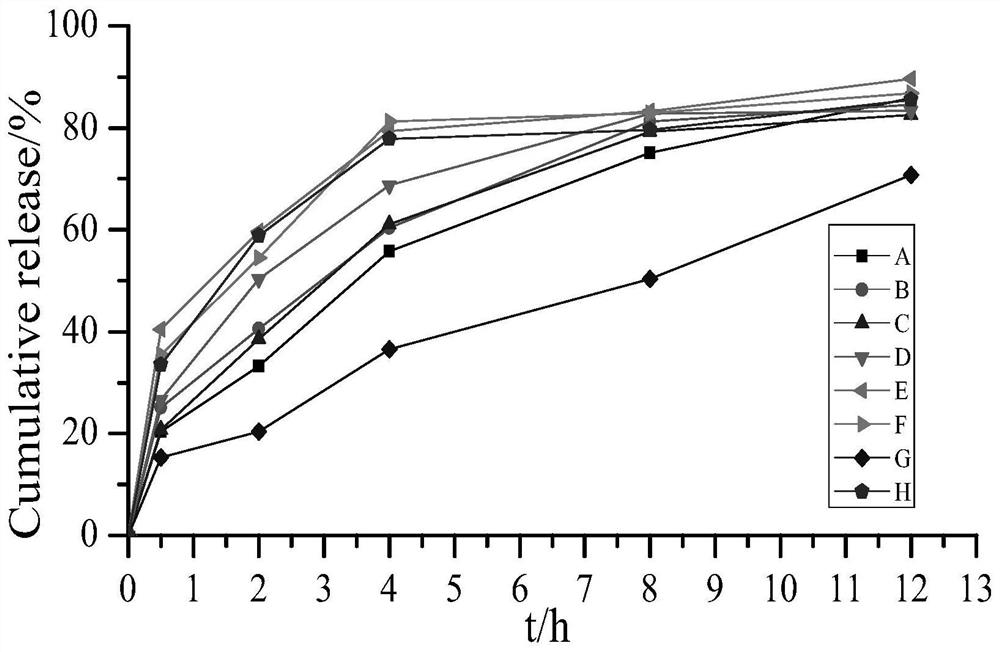
Examples
Embodiment 1
[0038] The formula of the huperzine A sustained-release pellets of the present embodiment is shown in Table 1.
[0039] Table 1
[0040]
[0041] The preparation method of the huperzine A sustained-release pellets of the present embodiment may further comprise the steps:
[0042] Dissolve binder and additives in purified water according to the formula and dosage in Table 1, and the resulting solution is used as wetting agent; then the wetting agent is mixed evenly with huperzine A, slow-release materials and pelleting auxiliary materials, and prepared into soft material; the soft material is prepared into pellets by an extrusion spheronizer, dried and sieved to obtain the product.
Embodiment 2
[0044] The formula of the huperzine A sustained-release pellets of the present embodiment is shown in Table 2.
[0045] Table 2
[0046]
[0047] The preparation method of the huperzine A sustained-release pellets of the present embodiment is the same as that in Example 1.
Embodiment 3
[0049] The formula of the huperzine A sustained-release pellets of the present embodiment is shown in Table 3.
[0050] table 3
[0051]
[0052]
[0053] The preparation method of the huperzine A sustained-release pellets of the present embodiment is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com