5-fluorouracil derivative and preparation method and application thereof
A technology of fluorouracil and reaction, applied in the field of 5-fluorouracil derivatives, can solve the problems of poor curative effect and inability of cancer drugs to penetrate, and achieve high anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
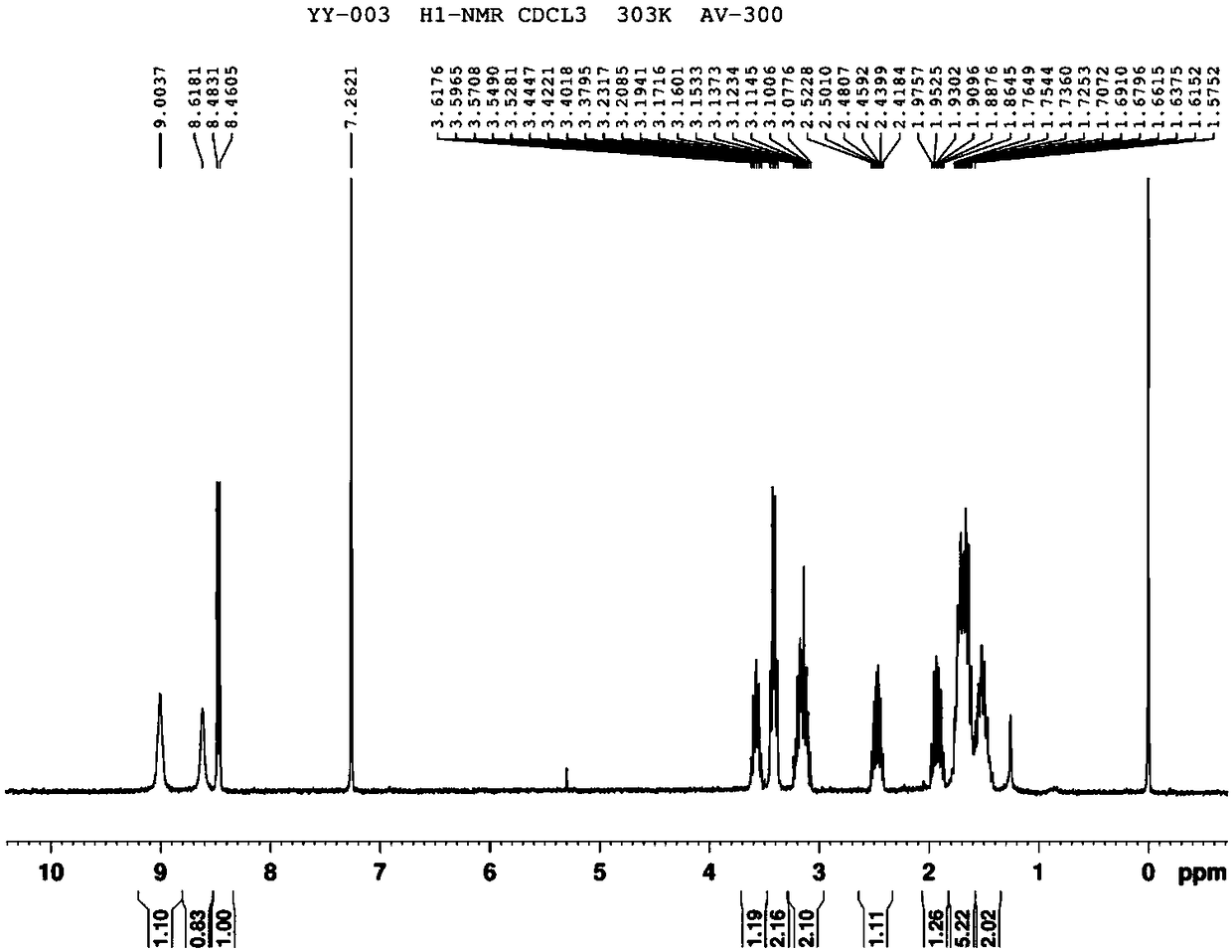
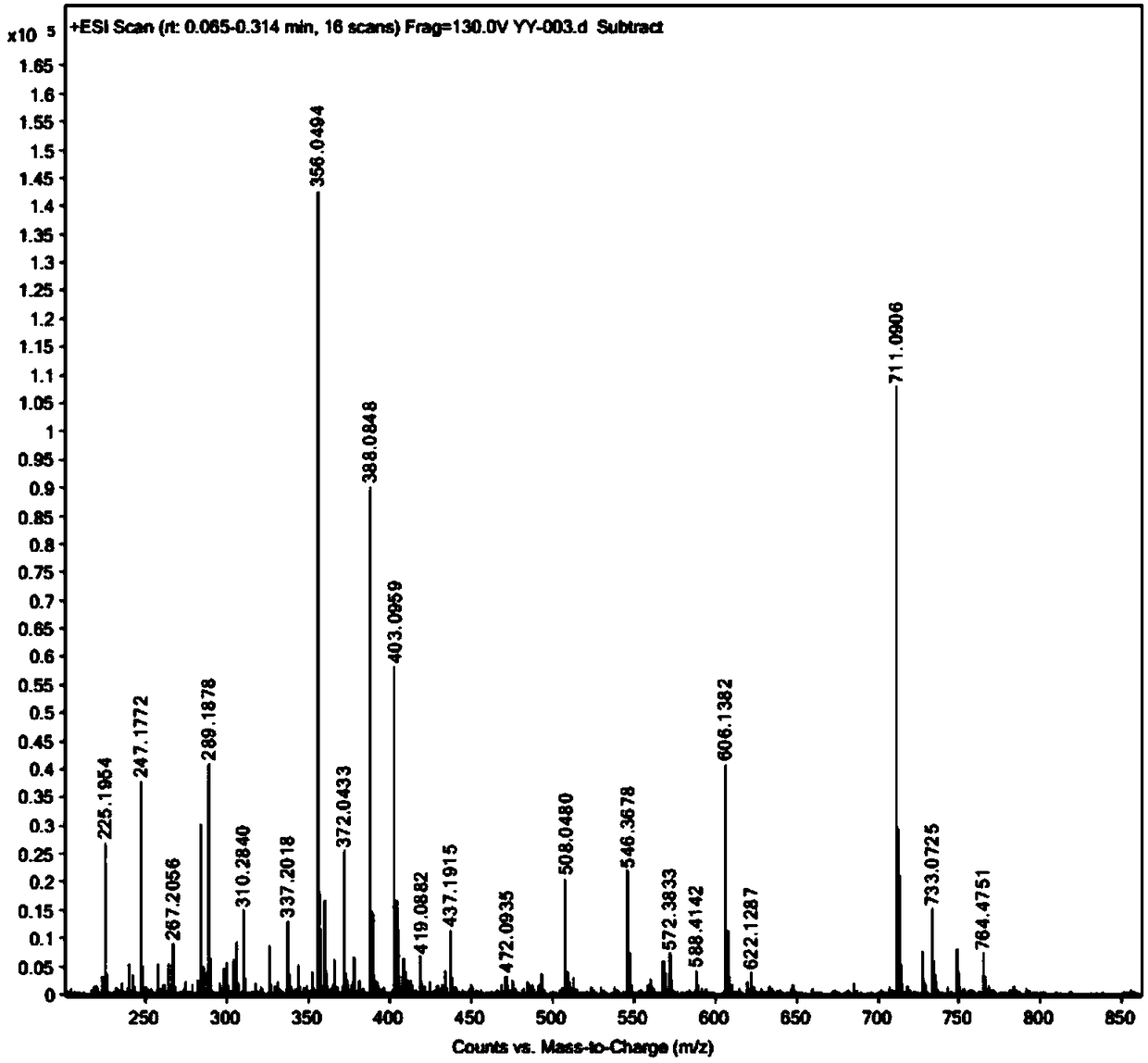
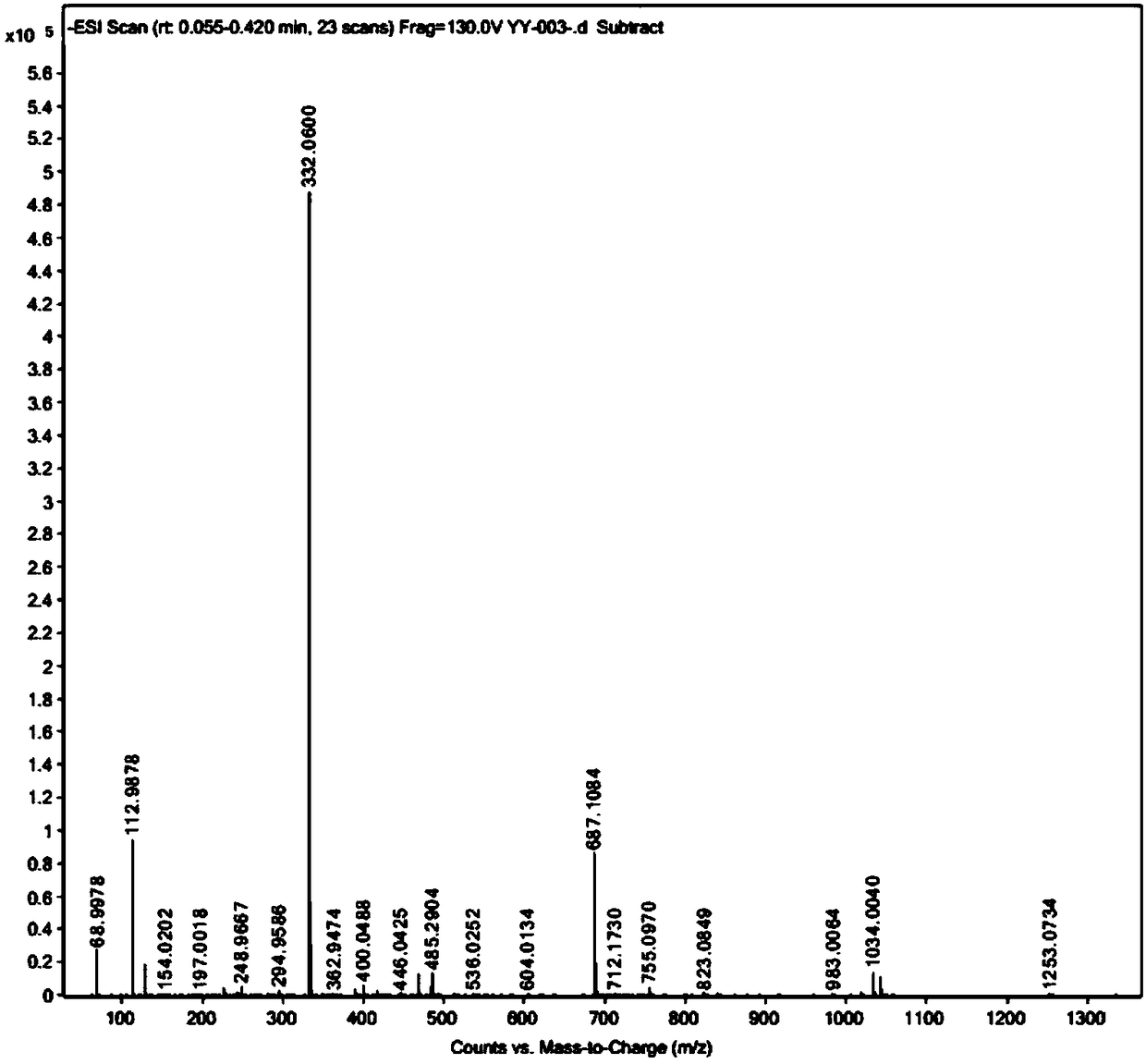
[0102] Example 1. Synthesis of 1-{4-[3-(1,2-dithiolane)]-butylamino}formyl-5-fluorouracil (YY-003)
[0103](1) Synthesis of 4-[3-(1,2-dithiolane)]-butyl isocyanate
[0104] The reaction formula is as follows:
[0105]
[0106] Experimental steps:
[0107] In a 100mL round bottom flask, add 1.0g (4.85mmol) lipoic acid and 10ml dry toluene, stir to dissolve, add 0.4g powdered 4A molecular sieve, and then add 2.105g (7.65mmol) phosphoric acid azide to the solution Diphenyl ester (DPPA) and 0.773g (7.65mmol) of triethylamine were stirred at room temperature until the reaction was complete (about 2 hours), and about 30ml of saturated NaHCO was added to the reaction solution 3 aqueous solution, stirred for 2-3 minutes, added ethyl acetate (30ml×3) for extraction three times, combined the organic phases, and washed with anhydrous Na 2 SO 4 After drying and column separation, 200-300 mesh silica gel was used as the stationary phase, and a mixture of dichloromethane and petroleu...
Embodiment 2
[0116] Example 2. Synthesis of 1-{4-[3-(1,2-dithiolane)]-butylamino}formyl-5-fluorouracil (YY-003)
[0117] (1) Synthesis of 4-[3-(1,2-dithiolane)]-butyl isocyanate
[0118] The reaction formula is as follows:
[0119]
[0120] In a 100mL round bottom flask, add 1.0g (4.85mmol) of lipoic acid and 5ml of acetone, stir to dissolve, add 0.539g (5.335mmol) of triethylamine, cool to 0 to -5°C in an ice-salt bath, and add to the solution A solution of 0.578 g (3.616 mmol) of ethyl chloroformate in acetone (2 ml) was slowly added dropwise, and after the drop was completed, stirring was continued at 0 to -5°C for 30 minutes. Then, 0.630 g (9.70 mmol) of an aqueous solution of sodium azide (2 ml) was slowly added dropwise to the solution, and stirring was continued at 0 to -5° C. for 30 minutes. Add 30ml of ice water to the solution, pour the mixture into a separatory funnel, add 50ml of toluene to the separatory funnel in four times to extract the reaction product, combine the to...
Embodiment 3
[0123] Example 3. Synthesis of 1-{4-[3-(1,2-dithiolane)]-butylamino}formyl-5-fluorouracil (YY-003)
[0124] (1) Synthesis of 4-[3-(1,2-dithiolane)]-butyl isocyanate
[0125] The reaction formula is as follows:
[0126]
[0127] In a 100ml flask, add 1.0g (4.85mmol) lipoic acid, 30ml chloroform and 2-3 drops of DMF, stir, 1.150g (9.7mmol) thionyl chloride (MW: 119), stir at room temperature for 4 hours under nitrogen protection , reacted at reflux for 1 hour, and removed the solvent and excess thionyl chloride under reduced pressure to obtain 4-[3-(1,2-dithiolane)]-pentanoyl chloride.
[0128] Add 0.630 g (9.70 mmol) of sodium azide and 10 ml of anhydrous tetrahydrofuran into a 100 ml flask, heat and stir until reflux. Dissolve the 4-[3-(1,2-dithiolane)]-pentanoyl chloride obtained above in 10ml of tetrahydrofuran and add it to the constant pressure dropping funnel. After reflux occurs, start to add 4-[3 -(1,2-Dithiolane)]-pentanoyl chloride was dissolved in 10ml of tetra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com