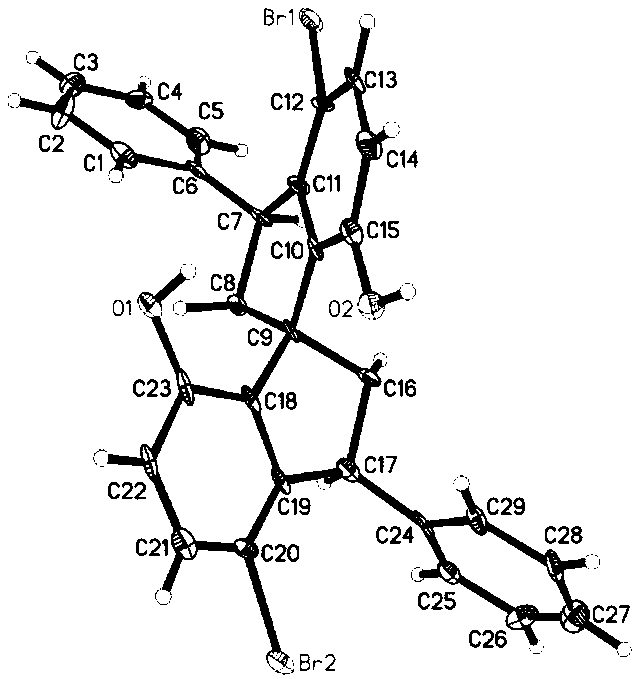
Asymmetric synthesis method for 3,3'-diaryl substituted chiral spiro bisphenol compound
A technology of spirocyclic diphenols and compounds, which is applied in the field of asymmetric synthesis of chiral spirocyclic diphenolic compounds, can solve problems such as limitations of spirocyclic partial modification, achieve high industrial production value, high yield, and enantioselective good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Using the following reaction scheme, taking the preparation of compound I-a as an example, the general preparation method of I described in the present invention is described in detail in this embodiment:
[0050]
[0051] Compound 1 (0.5g, 3.6mmol) and acetone (132μL, 1.8mmol) were added into the reaction flask, 40% NaOH aqueous solution (0.5mL) was slowly added dropwise at 0°C, moved to room temperature, and reacted for 8h. Pour the reaction solution into an ice-water bath, slowly add 2M HCl (4mL) dropwise, adjust the pH to 4-5, a solid precipitates, continue to stir for 0.5h, filter with suction, wash the filter cake with a large amount of water to obtain a crude product, which is recrystallized to obtain a yellow Solid 0.64g, yield 83%. Spectral data:
[0052] ESI-MS (m / z): 422.9; 1 H NMR (500MHz, Acetone) δ8.83(s, 2H), 8.05(d, J=15.9Hz, 2H), 7.55(d, J=8.7Hz, 2H), 7.41(d, J=2.8Hz, 2H ), 7.23 (d, J=15.9Hz, 2H), 6.94 (dd, J=8.7, 2.8Hz, 2H).
Embodiment 2
[0054] Compound 1 (0.5g, 3.6mmol) and acetone (132μL, 1.8mmol) were added into the reaction flask, 40% KOH aqueous solution (0.5mL) was slowly added dropwise at 0°C, moved to room temperature, and reacted for 8h. Pour the reaction solution into an ice-water bath, slowly add 2M HCl dropwise, adjust the pH to 4-5, a solid precipitates, continue to stir for 0.5h, filter with suction, wash the filter cake with a large amount of water to obtain a crude product, and obtain a yellow solid 0.58g after recrystallization , yield 75%.
Embodiment 3
[0056] Compound 1 (1.0 g, 7.2 mmol) and acetone (264 μL, 3.6 mmol) were added into the reaction flask, 40% NaOH aqueous solution (1.6 mL) was slowly added dropwise at 0° C., moved to room temperature, and reacted for 8 h. Pour the reaction solution into an ice-water bath, slowly add 2M HCl dropwise, adjust the pH to 4-5, a solid precipitates, continue to stir for 0.5h, filter with suction, wash the filter cake with a large amount of water to obtain the crude product, and obtain 1.0 g of a yellow solid after recrystallization , yield 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com