Human mesenchymal stem cell capable of delaying cell senescence and resisting malignant transformation and preparation method and application thereof
A technology of pluripotent stem cells and pluripotent stem cells, applied in the field of human mesenchymal stem cells and their preparation, can solve the problems of weakened application value, increased risk of gene mutation, and worrying safety, and achieves great application and promotion value and optimal tissue repair function. , the effect of good ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
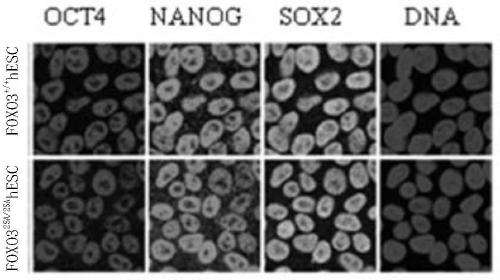
[0052] Example 1, Preparation of FOXO3 Enhanced Pluripotent Stem Cells
[0053] 1. Construction of recombinant plasmids
[0054] Insert the DNA molecule shown in Sequence 1 of the sequence listing between the SalI and SpeI restriction sites of the pAMHDAdGT8-4 vector to obtain a recombinant plasmid.
[0055] 2. Preparation of recombinant adenovirus
[0056] 1. Take the recombinant plasmid obtained in step 1, and digest it with PI-SceI to obtain a linearized plasmid.
[0057] 2. Introduce the linearized plasmid obtained in step 1 and the virus AdHPBGF35 into 116 cells, package the recombinant adenovirus, and then obtain recombinant adenovirus particles by ultra-high speed centrifugation.
[0058] 3. Infect target cells with recombinant adenovirus particles
[0059] Infect H9 cells (1×10 7 cells: 15btu virus), on the 2nd-4th day after infection, add G418 (25~400g / ml, product of Invitrogen Company) for positive selection, and on the 10th-13th day after infection, add 4μM Ganc...
Embodiment 2
[0070] Example 2. Preparation of FOXO3-enhanced mesenchymal stem cells and their functional testing
[0071] 1. Preparation of FOXO3-enhanced mesenchymal stem cells
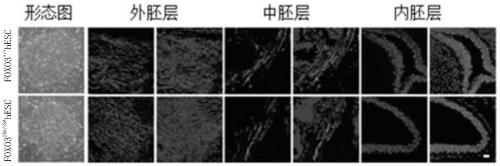
[0072] 1, with the FOXO3 that embodiment 1 obtains 2SA / 2SA hESC cells were differentiated into embryoid bodies to obtain embryoid bodies (EBs).
[0073] 2. Inoculate the embryoid body obtained in step 1 in a matrigel-coated 6-well plate and culture (using mesenchymal stem cell differentiation medium) until fibrous cells appear (about 2 weeks).
[0074] 3. After step 2 is completed, the cells are collected, digested with trypsin, and then inoculated into a new mesenchymal stem cell differentiation medium, and cultured for 2 weeks.
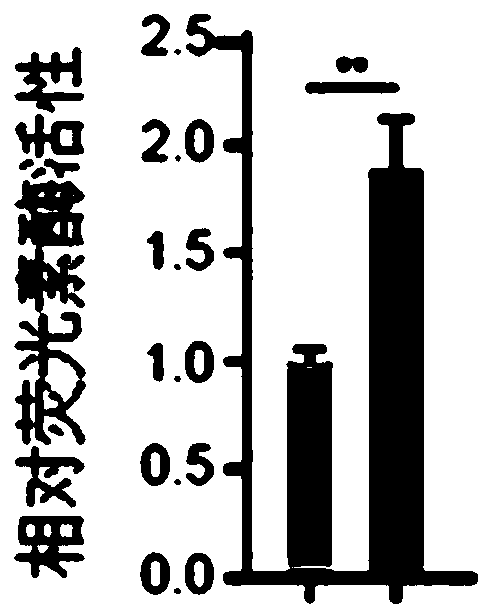
[0075] 4. After completing step 3, use flow cytometry to sort cells that are positive for CD73, CD90, and CD105, which are FOXO3-enhanced mesenchymal stem cells, also known as FOXO3 2SA / 2SA MSC cells.
[0076] 2. Preparation of control mesenchymal stem cells
[0077] Substitution ...
Embodiment 3
[0144] Example 3, Application of FOXO3 Enhanced Mesenchymal Stem Cells in Cell Transplantation Therapy
[0145] Test cells: FOXO3 prepared in Example 2 2SA / 2SA MSC cells or FOXO3 + / + MSC cells.
[0146] A lentiviral vector that overexpresses luciferase luciferase, referred to as luc lentiviral vector. Reference: SIRT6safeguards human mesenchymal stem cells from oxidative stress by coactivatingNRF2.Pan et.al,.Cell research.(2016)26:190-205; that is, "luciferase(Control)-expressed vector" in the literature.
[0147] Lentiviral packaging plasmid psPAX, referred to as plasmid psPAX, Adgene, Cat. No. 12260. Lentiviral packaging plasmid pMD2.G, referred to as plasmid pMD2.G, Adgene, Cat. No. 12259. 293T cells (human embryonic kidney cells): ATCC, CRL-3216.
[0148] In this example, the tested cells were used for cell transplantation therapy.
[0149] 1. Preparation of luciferase-labeled test cells.
[0150] 1. Use Lipofectamine 3000 kit (Invitrogen) to co-transfect 15 μg luc ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com