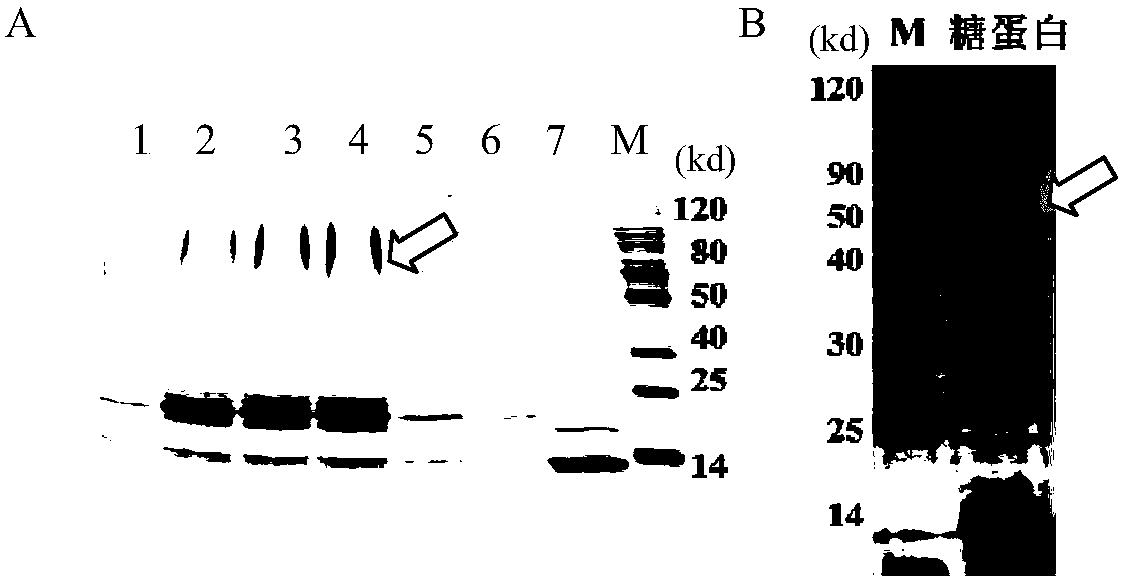Vaccine for preventing and treating diseases caused by Acinetobacter baumannii and preparation method thereof
A technology for Acinetobacter baumannii and vaccine, applied in the biological field, can solve the problems of low yield, difficulty in purification and quality control, poor product uniformity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
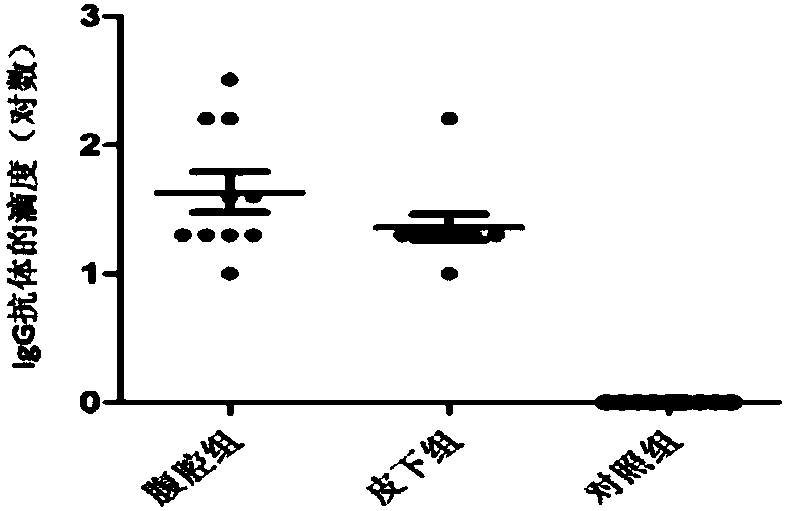
Examples
Embodiment 1
[0084] Embodiment 1, the construction of the recombinant vector expressing PglL and rCTB4573
[0085] 1. Construction of recombinant vector expressing PglL
[0086] The amino acid sequence of Neisseria meningitidis glycosyltransferase PglL is shown in SEQ ID No.3, and its coding sequence is shown in nucleotides 180-1994 of SEQ ID No.4. The 1st-6 nucleotides of SEQ ID No.4 are the XbaI recognition site, the 2475-2480th nucleotides are the SacI recognition sequence, the 2487-2492th nucleotides are the PstI recognition site, and the 2510-2492th nucleotides are the PstI recognition site. Nucleotide 2515 is the XhoI recognition site. The 105th-2240th nucleotides of SEQ ID No.4 are the sequence of the PglL expression cassette. In the PglL expression cassette, the expression of PglL is initiated by the tac promoter, and the expression cassette is named tacpglL. Wherein, the 105th-133rd nucleotide of SEQ ID No.4 is the sequence of tac promoter, and the 180th-1994th nucleotide of SEQ...
Embodiment 2
[0093] Example 2. Construction of glycoengineered Acinetobacter baumannii and detection of protein glycosylation
[0094] 1. Competent preparation of Acinetobacter baumannii electroporation
[0095]Cultivate Acinetobacter baumannii overnight at 37°C, subculture in low-salt LB liquid medium at a volume ratio of 1:100 and cultivate 50ml of Baumann's (low-salt LB liquid medium (500mL) formula: 5g peptone, 2.5g yeast powder , 2.5g NaCl, the balance is water, pH 7.0), 30 ℃ continue to cultivate to OD 600 When the value is 0.6, bathe in ice for 30 minutes, then centrifuge at 6000r / min and 4°C for 8 minutes to collect the bacteria, wash with high-pressure sterilized 10% glycerol four times, and finally resuspend the bacteria with 300 μL of 10% sterilized glycerol. Acinetobacter baumannii competent cells for electroshock transformation were obtained.
[0096] 2. Expression and glycosylation of fusion protein expression vector pET28tacpglL-tacrCTB4573 in Bowman
[0097] The pET28tac...
Embodiment 3
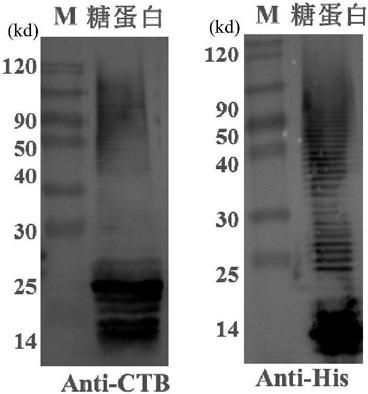
[0099] Example 3, Purification of Glycoengineering Acinetobacter baumannii Glycoprotein
[0100] 1. Pick the pET28tacpglL-tacrCTB4573 / Ab monoclonal clone of Example 2, inoculate it in 5ml LB liquid medium containing a final concentration of 50 μg / mL kanamycin, culture it overnight at 37°C, and pass on to LB liquid medium, cultured to OD at 37°C 600 At about 0.6, add IPTG with a final concentration of 1 mM, and cool down to 30° C. for induction for 12 hours to obtain a protein-inducing culture medium, and centrifuge to obtain protein-inducing cells.
[0101] 2. Sample pretreatment
[0102] Take 10g of the protein-induced bacteria in step 1, add 100ml of A1 solution (20mM pH7.5Tris-HCl, 0.5M NaCl, 10mM imidazole, adjust the pH to 7.0), ultrasonically break the bacteria (ultrasound for 4s and pause for 5s, and the cumulative ultrasonic time is 2h), Centrifuge with a centrifugal force of 12 000g to collect the supernatant, and then centrifuge the supernatant again with a centrif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com