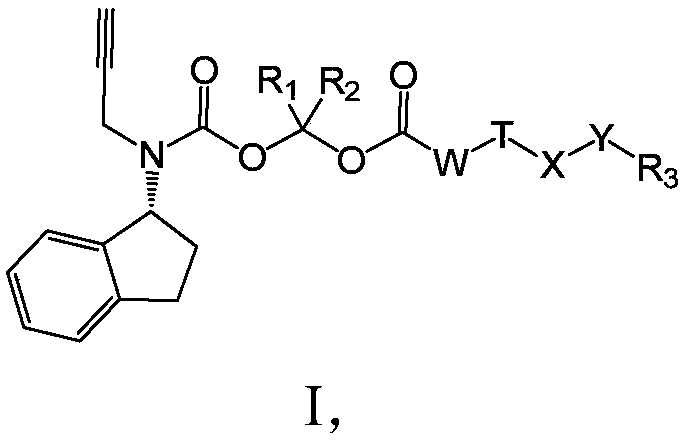
Long-acting Rasagiline prodrug as well as preparation method and application thereof
A prodrug and long-acting technology, applied in the field of long-acting rasagiline prodrug and its preparation, can solve the problems of forgetfulness, easy fusion of solid particles, and difficulty in controlling the drug release rate in the body, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
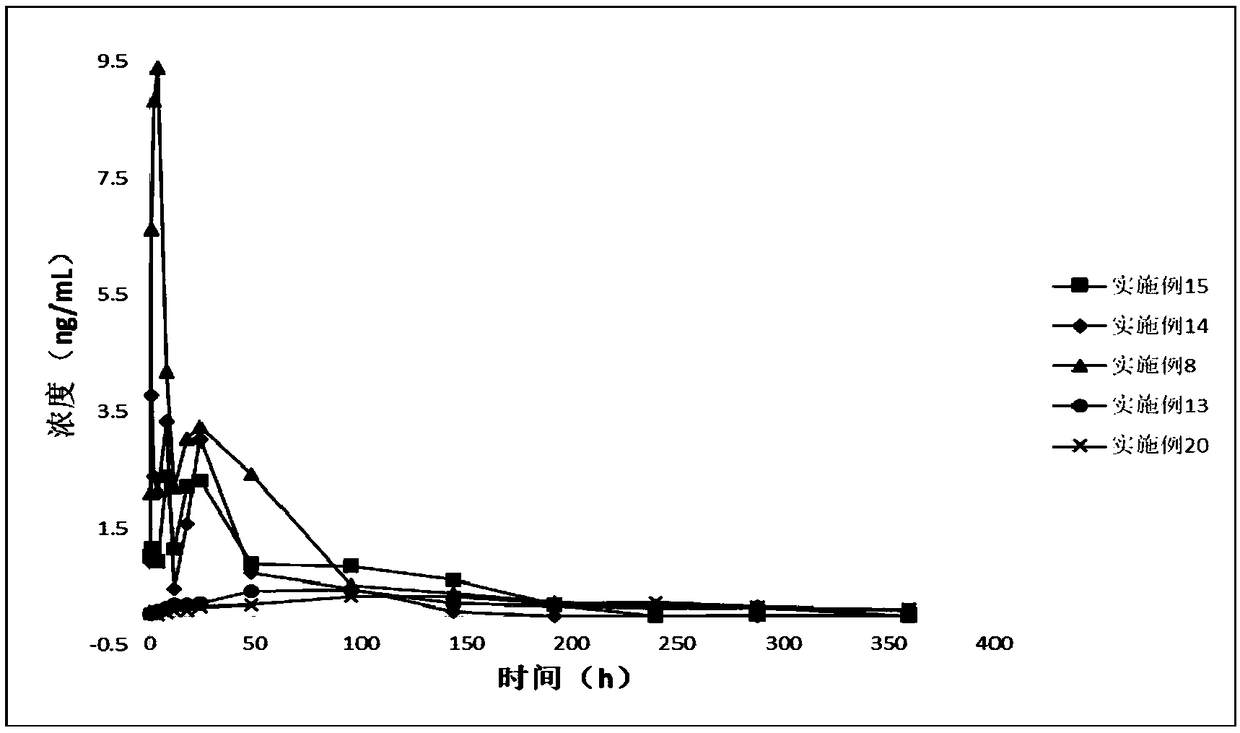
Image
Examples
Embodiment 1
[0086] Example 1 (Preparation of ((rasagiline-N-formyl)oxy)-methyl palmitate (see International Patent Application WO2013088255)
[0087]
[0088] Dissolve 1.0 g of rasagiline-N-carbonyl chloride methyl ester and 0.3 g of TBAI in THF / DMF (5+4 mL), add 1.5 g of potassium palmitate, and heat overnight at an external temperature of 57°C under argon protection. Stop the reaction, spin off THF, add isopropyl ether 30mL and stir for 10min, filter, and wash the filtrate with water (15mL filtrate) successively, saturated NaHCO 3 Washed, dried over anhydrous sodium sulfate, concentrated, and the concentrate was subjected to silica gel column chromatography (PE / EA=20:1-10:1) to obtain 0.76 g of a viscous product, yield: 41.53%.
[0089] 1 H-NMR (CDCl 3 ,400MHz)δ7.26(m,2H),7.19(m,2H),5.87(m,2.5H),5.78(m,0.5H),4.17(d,J=14.0Hz,0.5H),4.02( d,J=14.0Hz,0.5H),3.62(d,J=14.0Hz,0.5H),3.52(d,J=14.0Hz,0.5H),3.06(m,1H),2.86(m,1H) ,2.47(m,1H),2.36(m,2H),2.24(m,1H),2.21(s,0.5H),2.15(s,0.5H),1.6...
Embodiment 2
[0091] The preparation of embodiment 2 ((rasagiline-N-formyl) oxygen group)-naphthalene acetate methyl ester
[0092]
[0093] Dissolve 0.9 g of rasagiline-N-carbonyl chloride methyl ester and 0.25 g of TBAI in 15 mL of acetonitrile, add 0.92 g of potassium naphthalene acetate, and heat for 5 h at an external temperature of 60°C under the protection of argon. The reaction was stopped, the solvent was evaporated by rotary evaporation, 20 mL of ethyl acetate was added to dissolve, and saturated NaHCO 3 Wash (10mL×3), wash with saturated NaCl (10mL), anhydrous NaCl 2 SO 4 Dried, filtered, and spin-dried to obtain 1.38 g of crude product as light brown syrup. The crude product was recrystallized from methanol to obtain 0.65 g of pure rasagiline-N-carbomethyl naphthalene acetate as a white solid powder.
[0094] 1 HNMR (CDCl 3,400MHz)δ8.01(dd,J=14.8,8.4Hz,1H),7.84(m,2H),7.5(m,4H),7.22(m,3H),7.09(m,1H),5.90(m ,1.5H),5.82(dd,J=14.8,6.0Hz1H),5.54(t,J=8.0Hz,0.5H),4.16(s,1.07H)...
Embodiment 3
[0096] Example 3 (Preparation of ((rasagiline-N-formyl)oxy)-methyl stearate (see International Patent Application WO2013088255)
[0097]
[0098] Dissolve 2.0 g of rasagiline methyl carbochloride and 0.56 g of TBAI in 40 mL of DMF, add 3.2 g of potassium stearate, and heat the reaction overnight at an external temperature of 65° C. under the protection of argon. Stop the reaction, add 80mL of water, add 80mL of isopropyl ether for extraction, then use saturated NaHCO 3 Wash (30mL×2), saturated NaCl wash (30mL), anhydrous NaCl 2 SO 4 Dry, filter and spin dry to obtain 4.32g of crude product. The crude product was subjected to silica gel column chromatography (PE / EA=10:1) to obtain 3 g of the product as light yellow syrup with a yield of 51.55%.
[0099] 1 HNMR (CDCl 3 ,400MHz)δ7.25(m,2H),7.19(m,2H),5.87(m,2.5H),5.66(m,0.5H),4.12(d,J=17.6Hz,0.5H),3.98( d,J=17.6Hz,0.5H),3.57(d,J=17.6Hz,0.5H),3.47(d,J=17.6Hz,0.5H),3.06(m,1H),2.85(m,1H) ,2.46(m,1H),2.37(m,2H),2.24(m,1H),2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com