Synthetic method and application for water-soluble nitrogen-iron co-doped carbon dot
A co-doping and water-soluble technology, which is applied in the field of nanomaterial science and application, can solve the problems of complex process, low carbon dot fluorescence quantum yield, and high carbon dot fluorescence quantum yield, and achieve simple equipment and complex equipment operation , Highly reproducible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 Preparation of nitrogen-iron co-doped carbon dots
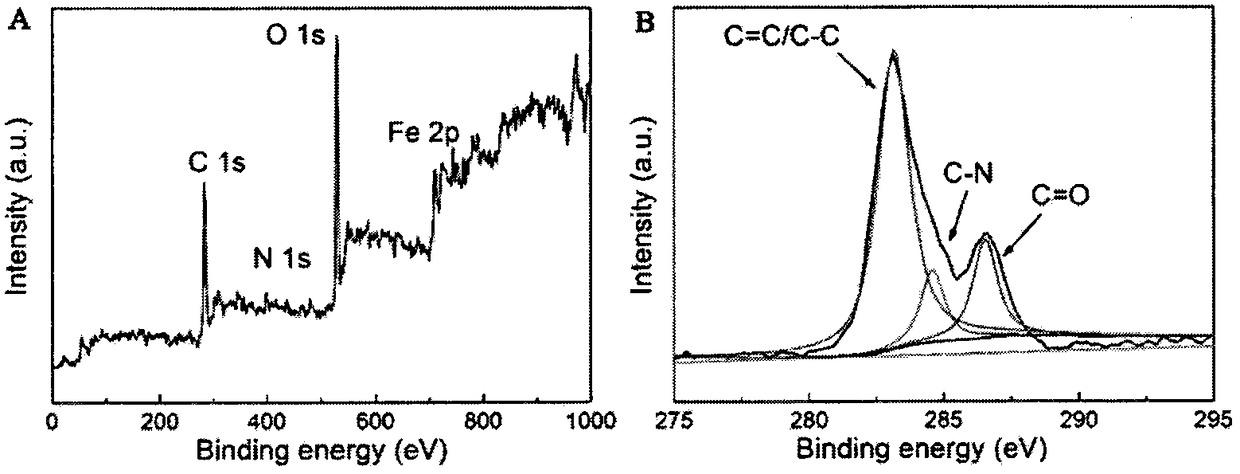
[0043] Dissolve 1g of ferric ammonium citrate and 0.8g of urea in 30mL of deionized water, transfer it to a 100mL reaction kettle, seal it and perform a hydrothermal reaction at 180°C for 6 hours, cool to room temperature, and use an appropriate amount of NaOH to obtain a reddish-brown precipitate. The precipitate was removed by rotating speed centrifugation to obtain a light yellow transparent solution, which was neutralized to neutral with HCl solution, dialyzed for 48 hours, and dried in a freeze dryer for 48 hours to obtain carbon dot powder co-doped with nitrogen and iron.
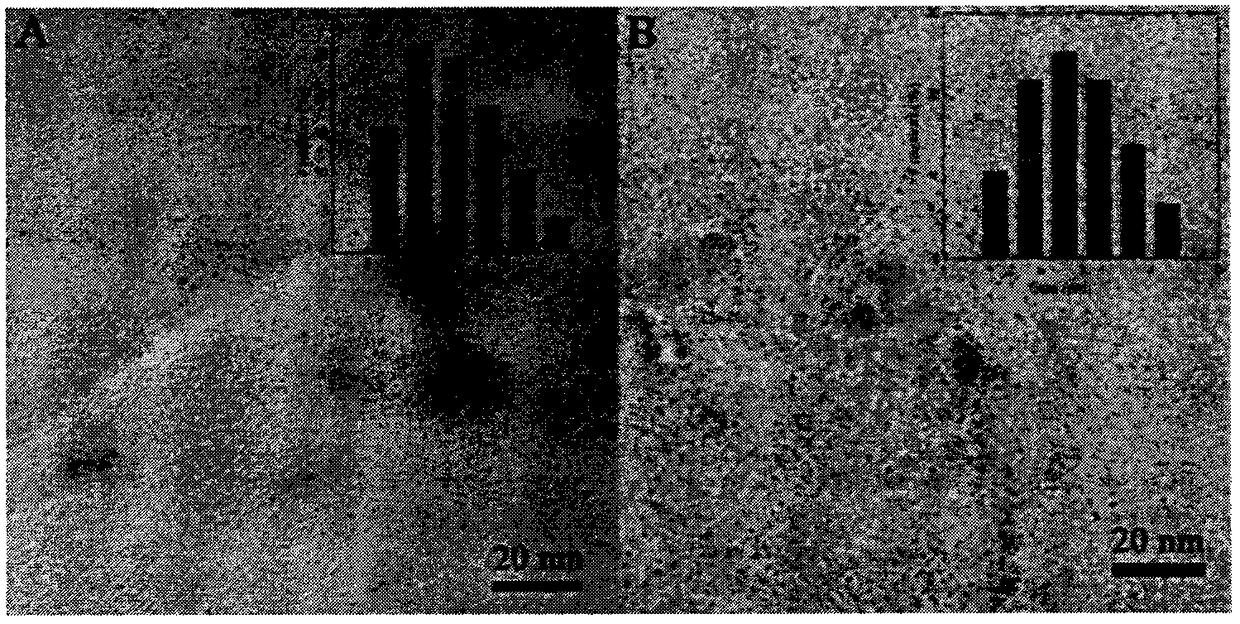
[0044] see figure 1 A, is the scanning electron micrograph of the carbon dots synthesized under this condition and the carbon dot size distribution. It can be seen from the figure that the obtained nitrogen-iron co-doped carbon dots have a spherical structure with uniform size and an average particle size of 4.5 nm, good dispersi...
Embodiment 2
[0048] Embodiment 2 Preparation of nitrogen-iron co-doped carbon dots
[0049] Dissolve 1g of ferric ammonium citrate in 30mL of deionized water, transfer it to a 100mL reaction kettle, seal it, and conduct a hydrothermal reaction at 180°C for 6 hours, cool to room temperature, use an appropriate amount of NaOH to obtain a reddish-brown precipitate, and centrifuge at 7000rpm to remove the precipitate , to obtain a light yellow transparent solution, which was neutralized to neutral with HCl solution, dialyzed for 48 h, and dried in a freeze dryer for 48 h to obtain nitrogen-iron co-doped carbon dot powder.
[0050] see figure 1 B, is the scanning electron micrograph of the carbon dots synthesized under this condition and the carbon dot size distribution diagram. It can be seen from the figure that the obtained nitrogen-iron co-doped carbon dots have a spherical structure with uniform size and an average particle size of 2.6nm, good dispersion, no obvious coagulation.
Embodiment 3
[0051] Example 3 An ion detection application evaluation experiment with water-soluble nitrogen-iron co-doped carbon dots in this experimental mode.
[0052] Test 1: Dissolve 1g of ferric ammonium citrate and 0.8g of urea in 30mL of deionized water, transfer it to a 100mL reaction kettle, seal it and perform hydrothermal reaction at 180°C for 6h, cool to room temperature, and use an appropriate amount of NaOH to obtain a reddish-brown precipitate , centrifuged at 7000rpm to remove the precipitate to obtain a light yellow transparent solution, which was neutralized to neutral with HCl solution, dialyzed for 48 hours, and dried in a freeze dryer for 48 hours to obtain carbon dot powder co-doped with nitrogen and iron .
[0053] Experiment 2: Use a fluorescence spectrophotometer to detect the effect of the fluorescence intensity of the carbon dots obtained in Experiment 1 on the pH value of the solution. Figure 5 Fluorescence of carbon dots measured at different pH values. It c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com