Astaxanthin nanometer preparation prepared by electrostatic spraying method and preparation method thereof
A nano-preparation and electrostatic spraying technology, which is applied in the direction of anti-inflammatory agents, anti-toxic agents, pill delivery, etc., can solve the problems of preparation technology blank, achieve good solubility, increase release speed, and improve oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 astaxanthin nano preparation
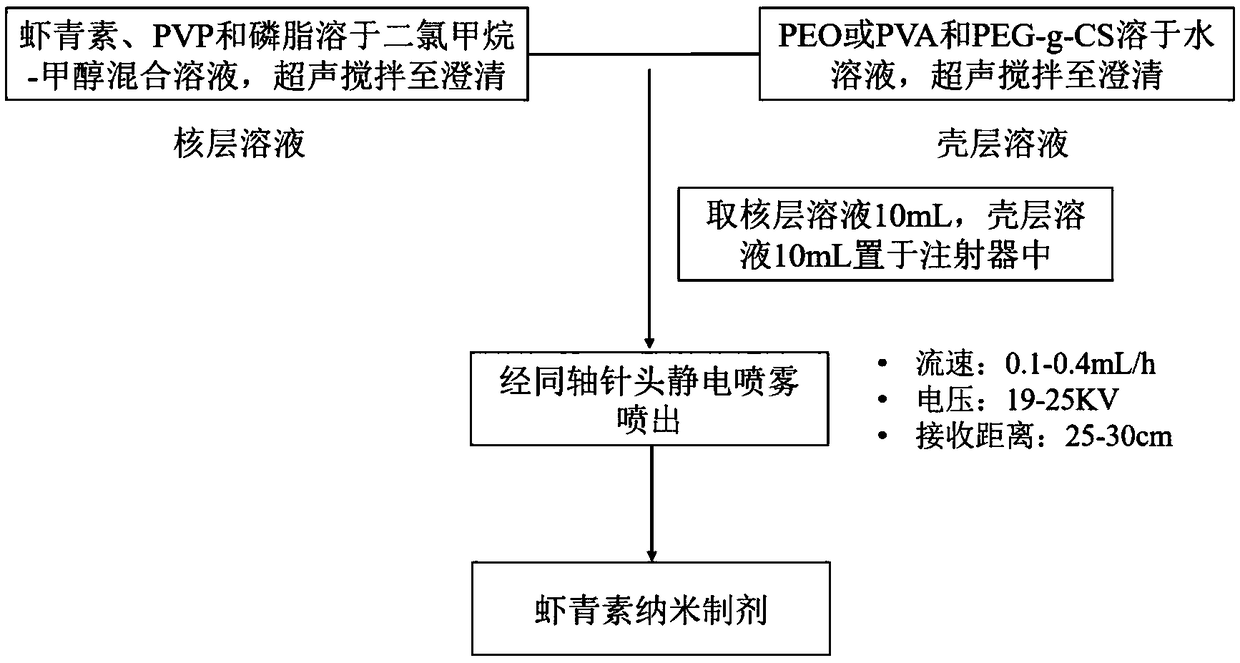
[0035] (1) Accurately weigh 10 mg of astaxanthin and 20 mg of phospholipid, place them in 10 mL of dichloromethane / methanol solvent (volume ratio 2:8), wherein the total concentration of absorption-promoting components in the nuclear layer solution is 0.2%, and ultrasonically disperse for 1-2 After 20 minutes, stir magnetically for 20 minutes to mix evenly to a transparent and clear solution to obtain a core layer solution; dissolve 40 mg of PEG-g-CS and 20 mg of PEO in 10 mL of distilled water so that the total concentration of the shell layer carrier is 0.6%, and disperse ultrasonically for 1-2 minutes After that, magnetically stir for 20 minutes to mix evenly until a transparent and clear solution is obtained to obtain a shell solution.
[0036] (2) Put 10mL each of the core layer solution and the shell layer solution into the syringe, and slowly output it from the constant flow pump. The spray speed of the core layer solut...
Embodiment 2
[0037] Embodiment 2 astaxanthin nano-preparation
[0038] (1) Accurately weigh 10 mg of astaxanthin, 40 mg of phospholipid, 40 mg of PVP K30, and 40 mg of PEG 6000, and place them in 10 mL of dichloromethane / methanol solvent (volume ratio 3:7), in which the hydrophilic polymer carrier and The total concentration of absorption-promoting ingredients is 1.2%. After ultrasonic dispersion for 1-2 minutes, stir magnetically for 20 minutes and mix evenly until a transparent and clear solution is obtained to obtain a nuclear layer solution; PEG-g-CS 200mg and PEO 60mg are dissolved in 10mL distilled water to The total concentration of the shell layer carrier is 2.6%. After ultrasonic dispersion for 1-2 minutes, the mixture is mixed uniformly by magnetic stirring for 20 minutes until a transparent and clear solution is obtained to obtain a shell layer solution.
[0039] (2) Put 10 mL each of the core layer solution and the shell layer solution into the syringes, and slowly output it fr...
Embodiment 3
[0040] Embodiment 3 astaxanthin nano-preparation
[0041] (1) Accurately weigh 10 mg of astaxanthin, 30 mg of phospholipid, 20 mg of PVP K30, and 20 mg of PEG 4000, and place them in 10 mL of dichloromethane / methanol solvent (volume ratio 4:6), in which the hydrophilic polymer carrier and The total concentration of the absorption-promoting ingredients is 0.7%. After ultrasonic dispersion for 1-2 minutes, stir magnetically for 20 minutes and mix evenly to a transparent and clear solution to obtain a nuclear layer solution; dissolve 150 mg of PEG-g-CS and 60 mg of PEO in 10 mL of distilled water to make The total concentration of the shell carrier is 2.1%. After ultrasonic dispersion for 1-2 minutes, the mixture is mixed uniformly by magnetic stirring for 20 minutes until a transparent and clear solution is obtained to obtain a shell solution.
[0042] (2) Put 10mL each of the core layer solution and the shell layer solution into the syringe, and slowly output it from the consta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com