Synthesis method of sofosbuvir intermediate
A synthesis method and intermediate technology, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of sodium hypochlorite sensitization, nail thinning, hair loss, etc., achieve huge industrial production prospects, save production costs, and increase the effect of reaction intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
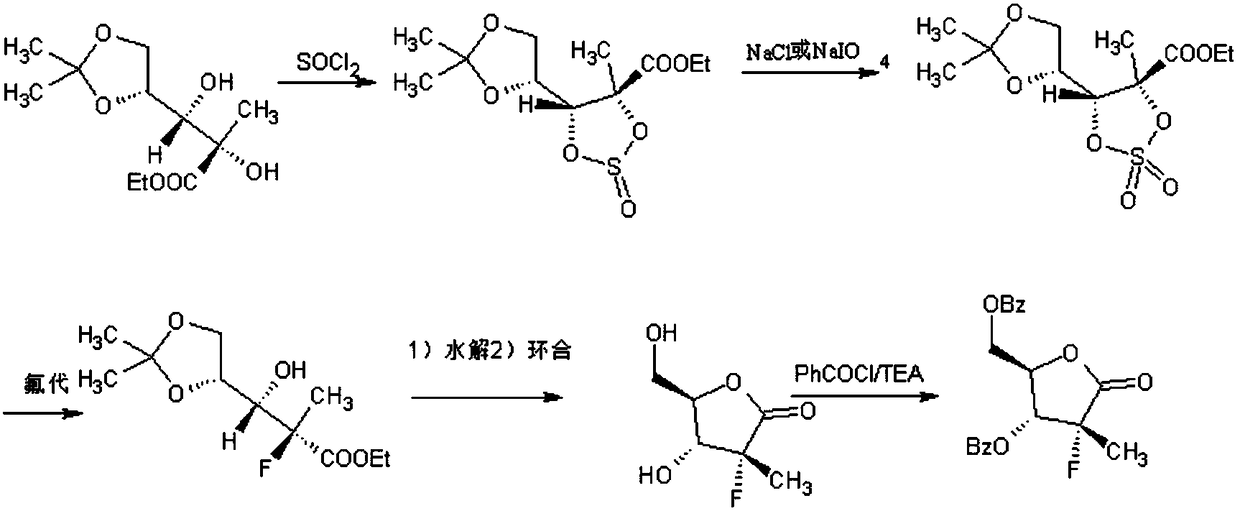
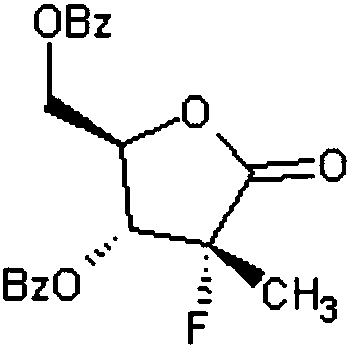
[0025] A kind of synthetic method of Sofosbuvir intermediate (I), concrete steps are as follows:
[0026] Step 1, the synthesis of compound (Ⅲ): under the condition of 0-5°C, add 24.8g (0.1mol) of compound (Ⅱ), 200g of dichloromethane and 20.2g (0.2mol) of triethylamine into a dry three-necked flask, and stir To 0°C, slowly add thionyl chloride 17.9g (0.15mol), react for 30min, add ice water 100g, dichloromethane 200g, stir for 30min, separate the organic layer, then use 100g of water and 20% The organic layer was washed with brine to obtain a dichloromethane solution of compound (Ⅲ).
[0027] Step 2, the synthesis of compound (Ⅳ): Add 1.65 g (0.05 mol) of sodium tungstate dihydrate to the dichloromethane solution of compound (Ⅲ) in step 1, and add 28.3 g of 30% hydrogen peroxide dropwise at 23°C (0.25mol), continue the reaction for 9h until the compound (Ⅲ) disappears, filter and recover sodium tungstate dihydrate, and wash the filtrate with 50g of 10% sodium bisulfite and 5...
Embodiment 2
[0032] A kind of synthetic method of Sofosbuvir intermediate (I), concrete steps are as follows:
[0033] Step 1, the synthesis of compound (Ⅲ): under the condition of 0-5°C, add 22.6g (0.09mol) of compound (Ⅱ), 180g of dichloromethane and 18.2g (0.18mol) of triethylamine into a dry three-necked flask, and stir To 0°C, slowly add thionyl chloride 15.5g (0.13mol), react for 20min, add ice water 100g, dichloromethane 200g, stir for 30min, separate the organic layer, then use 100g of water and 20% The organic layer was washed with brine to obtain a dichloromethane solution of compound (Ⅲ).
[0034]Step 2, the synthesis of compound (Ⅳ): Add 15 g (0.045 mol) of sodium tungstate dihydrate to the methylene chloride solution of compound (Ⅲ) in step 1, and add 26.4 g of 35% hydrogen peroxide dropwise at 20° C. ( 0.27mol), continue the reaction for 8 hours until compound (Ⅲ) disappears, filter and recover sodium tungstate dihydrate, and wash the filtrate with 50 g of 10% sodium bisulfi...
Embodiment 3
[0039] A kind of synthetic method of Sofosbuvir intermediate (I), concrete steps are as follows:
[0040] Step 1, synthesis of compound (Ⅲ): under low temperature conditions, add 29.76g (0.12mol) of compound (Ⅱ), 230g of dichloromethane and 21.2g (0.21mol) of triethylamine into a dry three-necked flask, and stir to 0°C , slowly add thionyl chloride 18.6g (0.156mol), react for 40min, add ice water 100g, dichloromethane 200g, stir for 30min, separate the organic layer, then wash with 100g of water and 20% brine respectively organic layer to obtain a dichloromethane solution of compound (Ⅲ).
[0041] Step 2, synthesis of compound (Ⅳ): add 1.75 g (0.053 mol) of sodium tungstate dihydrate to the dichloromethane solution of compound (Ⅲ) in step 1, and add 29.9 g of 25% hydrogen peroxide dropwise at 25°C (0.22mol), continue to react for 10h until compound (Ⅲ) disappears, filter and recover sodium tungstate dihydrate, and wash the filtrate with 50g of 10% sodium bisulfite and 50g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com