Novel almoxicillin sodium and clavulanate potassium compound powder preparation for injection and technology for preparing same
A technology of amoxicillin sodium clavulanate potassium and amoxicillin sodium, applied in the field of compound antibiotics and preparation process, new injection packaging form, can solve the problems that no one has developed injection preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012] Example 1, preparation and storage of eutectic
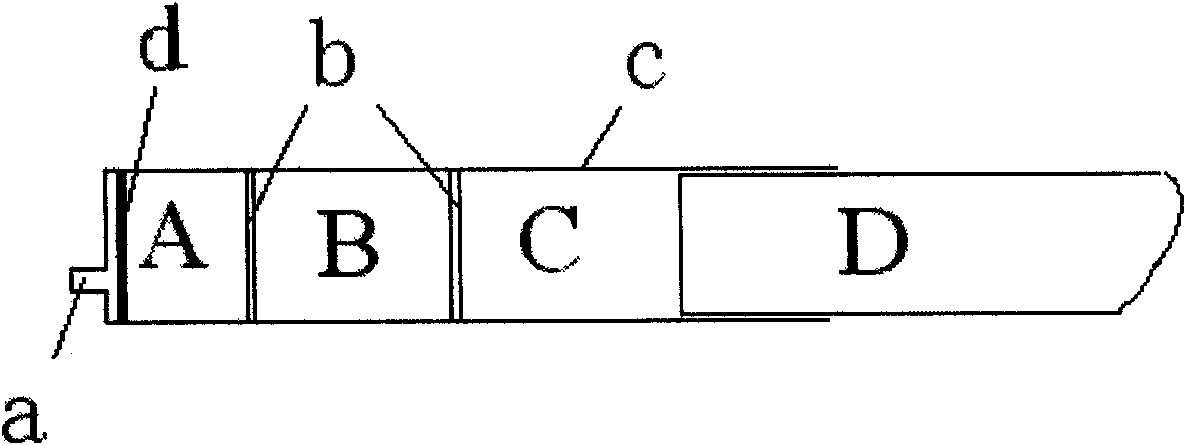
[0013] First heat the glass-lined reaction pot, drive out the air, fill it with nitrogen with a humidity below 0.2%, and cool it down. Put 90 L of anhydrous methanol / absolute ethanol (volume ratio 1:30) mixed solution into a glass-lined pot, then put 35 kg of amoxicillin sodium and 5 kg of potassium clavulanate powder into the above solution, and put it under the continuous flow of nitrogen. After heating to 25±2°C, stirring, mixing and dissolving, cooling to 5°C to crystallize, removing the solution under aseptic conditions to obtain a sterile 7:1 eutectic of amoxicillin sodium / potassium clavulanate, the crystal particles are complete , containing low active water. Vacuum dry under a sterile nitrogen flow until the moisture content is below 0.5%. And wash the disposable syringes in the GMP injection powder filling line, and dry the syringes under the clean nitrogen flow, fill the filling machine and syringes (Part A) w...
example 2
[0014] Example 2. Quality research on eutectic stability:
[0015] Properties: The properties of the trial sample are off-white powder.
[0016] Identification: The retention time of the high-performance liquid chromatography peak is compared with that of the reference substance, and compared with the standard substance, it is a qualified product.
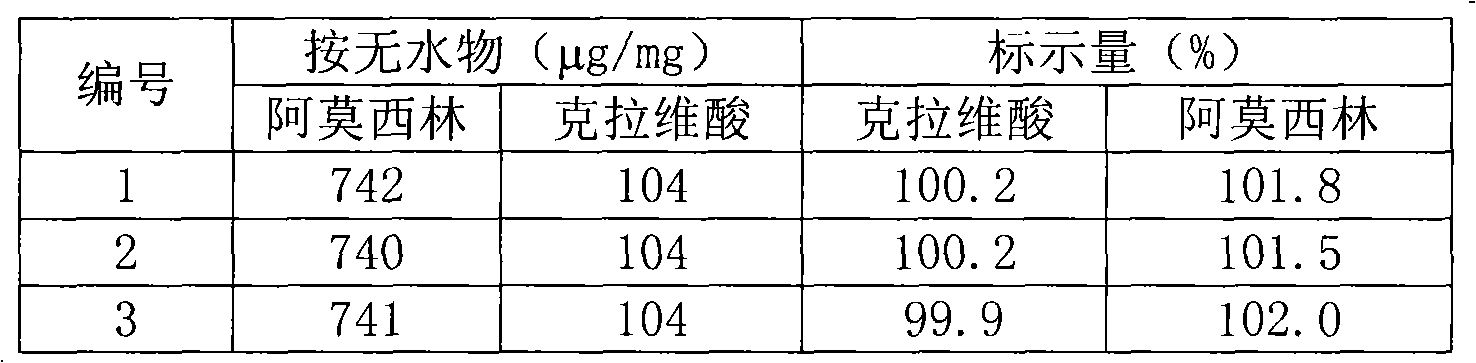
[0017] Alkalinity: the pH value of the aqueous solution of this product is 9.0, the quality standard of amoxicillin sodium, its alkalinity range is 8.0~10.0 (aqueous solution, 0.1g / ml), the pH value range of the quality standard of clavulanate potassium is 6.0~ 8.0. The alkalinity range of the trial-produced samples is 8.0-10.0, which is a qualified sample.
[0018] The clarity of the solution: the clarity of the trial sample solution does not exceed the No. 2 turbidity standard solution, which is a qualified sample.
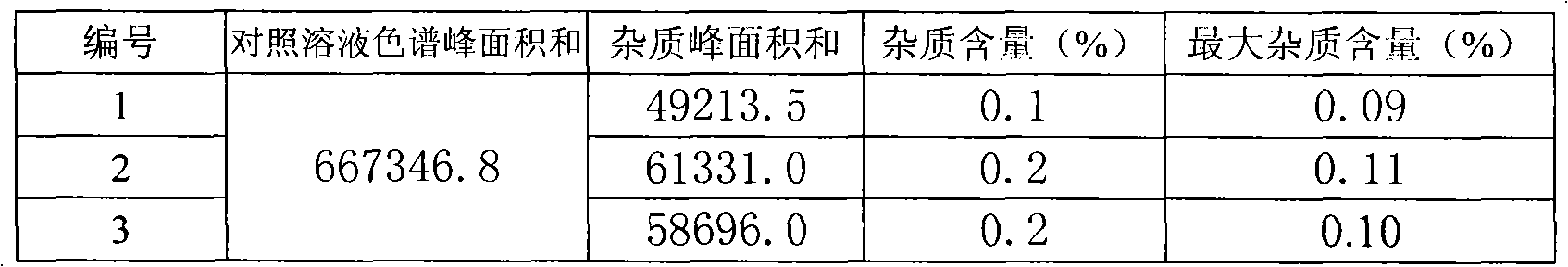
[0019] Related substances: The determination method of related substances is defined as the HPLC reference subs...
example 3
[0040] Example three, the pharmaceutical test of co-crystal
[0041] 1. The protective effect of amoxicillin sodium clavulanate potassium (7:1) compound preparation on mice infected with Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus in vivo.
[0042] Drugs (1) Amoxicillin Sodium for Injection (AMPC). (2) Amoxicillin Sodium Clavulanate Potassium for Injection 7:1 ratio (AMPC / CA). (3) The above-mentioned medicines of Sigeer (amoxicillin sodium and clavulanate potassium 5:1) were all stored in the freezer, and diluted with sterile water for injection before use to the required concentration.
[0043] Animal ICR mice, weighing 18-22g, male and female.
[0044] Experimental strains Three enzyme-producing bacteria strains, Escherichia coli (3128), Pseudomonas aeruginosa (3302) and Staphylococcus aureus (3711), all of the above strains were clinical isolates.
[0045] Protective agent highly active dry yeast, commercially available.
[0046] Experimental meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com