1,2,3-triazole tubulin polymerization inhibitor and synthesis method and application thereof
A technology of polymerization inhibitor and triazole, which is applied in the field of anti-tumor pharmaceutical chemistry, can solve the problems of high toxicity and achieve the effect of high yield and simple and efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of Embodiment 1 General Formula (6a~6l) (1) Preparation of Compound (4):
[0022] (1) In an ethanol solvent at room temperature, add 3,4,5-trimethoxyaniline (1), 4-methoxybenzyl chloride, and sodium carbonate to react, then add chloroacetyl chloride to react to obtain compound (4);
[0023] (2) Preparation of compound (5):
[0024] In the ethanol solvent, add potassium phosphate, compound (4) and sodium azide, stir and react at 60°C to obtain compound (5);
[0025] (3) Preparation of compound (6a~6l): Add compound (5) (1.93g, 5mmol) and different alkynyl compounds (6mmol) into 10mL acetone / water (5ml / 5ml) to dissolve, and finally add copper sulfate pentahydrate (1mmol ) and sodium ascorbate (0.5 mmol), stirred overnight at room temperature. TLC monitors the reaction process. After the reaction is over, add distilled water to the system, then extract 6 times with dichloroethane, then back-extract the dichloroethane phase with saturated brine for 3 times, ea...
Embodiment 2
[0038] The antitumor activity determination of the above-mentioned compound of embodiment 2:
[0039] The compounds used in the screening are all synthesized and purified by the present invention; sample stock solution: weigh 1-2mg of the sample and place it in a 2mLEP tube, then prepare a solution with DMSO, store it at 4°C, and use the culture medium according to the required concentration during the experiment dilution. Take the cells in the logarithmic growth phase, digest and count them, adjust the cell density with the medium, inoculate 4000-5000 cells / well into a 96-well plate, 150 μL per well, cultivate for 24 hours, discard the medium, and add the culture medium Base-diluted drugs (50 μg / mL, 100 μg / mL), 6 replicate wells were set up for each concentration, and a blank control group and a negative control group were also set up. After 72 hours of drug action, add 20 μL MTT to each hole, continue to cultivate for 4 hours, absorb the liquid, add 150 mL of DMSO, shake ev...
Embodiment 3
[0043] The Tubulin polymerization inhibition activity assay of embodiment 3 compound 6c:
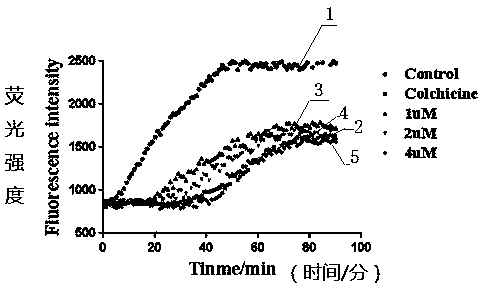
[0044] Resuspend the extracted tubulin in ice-cold G-PEM buffer (80mM PIPES pH 5.9, 5mM MgCl 2 , 1mMEGTA, 1mMATP, 5% (v / v) glycerol), take 100ul and add it to a 96-well plate containing 100ul compound 6c, the final concentration of tubulin is 5.6g / L, and the drug concentration is set to 0uM, 1uM, 2uM, 4uM Four gradients, the samples were mixed well, the aggregation of tubulin was detected by spectrophotometer, the interval was 5min, the total was 60min, IC 50 Values were calculated at 30 minutes using GraphPad software. IC of the tubulin polymerization inhibitory activity of compound 6c 50 The value is 2.17μM, indicating that compound 6c can indeed bind to tubulin and inhibit its polymerization. The results are shown in figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com