Boron-containing compound, preparation method thereof and application of boron-containing compound
A technology of boron compounds and compounds, applied in the field of boron-containing organic light-emitting materials and their preparation, can solve problems such as restricting the application of TADF materials, affecting the color purity of materials, and increasing molecular charge transfer, so as to achieve short-wavelength light emission and good color purity , weaken the effect of luminous red shift
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
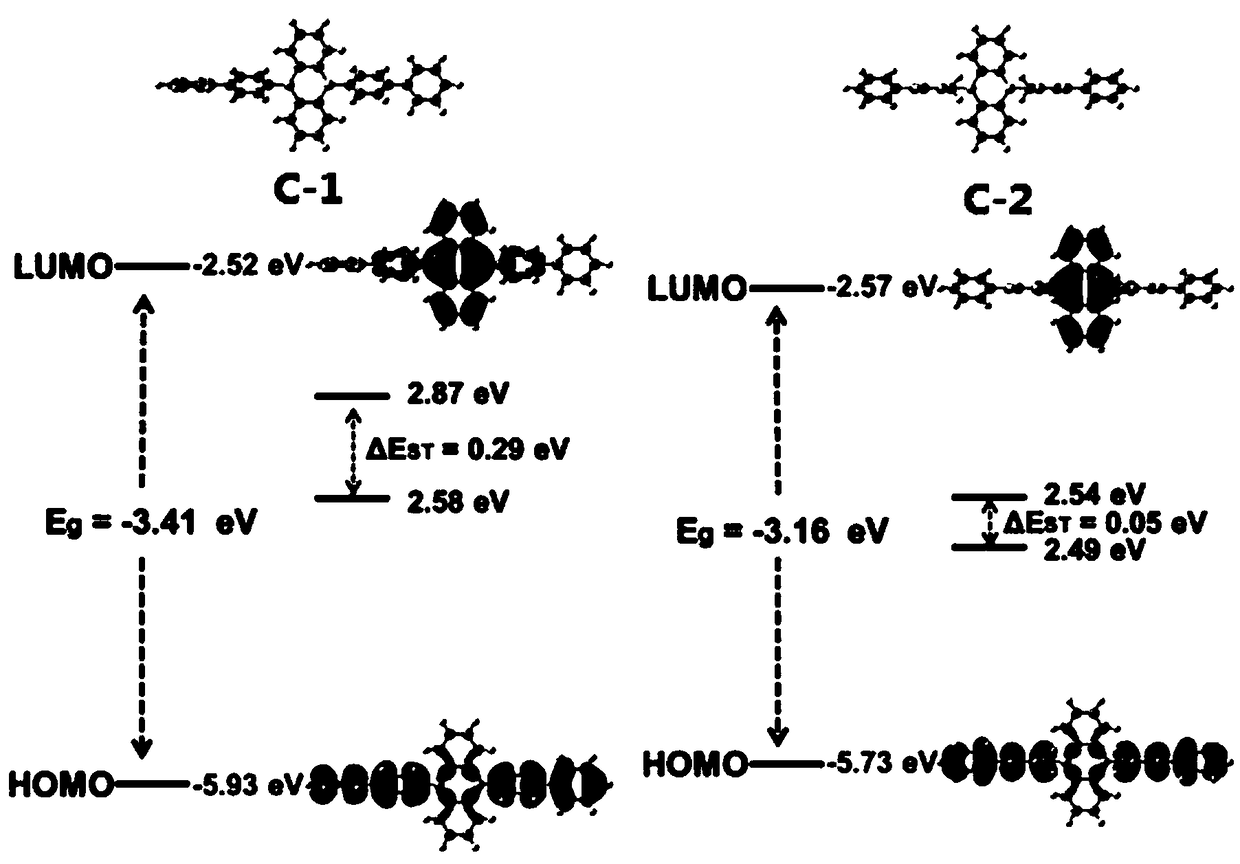
[0048] This embodiment provides a boron-containing compound, which has the structure shown in the following formula C-1:
[0049]
[0050] The synthetic route of the boron-containing compound shown in formula C-1 is as follows:
[0051]
[0052] The preparation method of the boron-containing compound shown in formula C-1 specifically comprises the following steps:
[0053] Under the protection of nitrogen, add 4.6g (20mmol) of compound M-2, 500mL of diethyl ether, dropwise add 18.75mL of n-butyl lithium (1.6M, 30mmol) at -78°C, react at low temperature for 30min, and slowly warm up to room temperature for 0.5 h, cooled to -78°C again and added 300mL of compound M-1 (3.3g, 10mmol) in toluene solution dropwise, slowly rose to room temperature and reacted for 10h, added aqueous ammonium chloride to quench the reaction, extracted with dichloromethane, and spun The solvent was evaporated and passed through a silica gel column to obtain 3.6 g of solid compound C-1 (yield 76%)...
Embodiment 2
[0056] This embodiment provides a boron-containing compound, which has the structure shown in the following formula C-3:
[0057]
[0058] The synthetic route of the boron-containing compound shown in formula C-3 is as follows:
[0059]
[0060] The preparation method of the boron-containing compound shown in formula C-3 specifically comprises the following steps:
[0061] Under the protection of nitrogen, add 2.3g (10mmol) of compound M-2, 500mL of diethyl ether, dropwise add 6.25mL of n-butyllithium (1.6M, 10mmol) at -78°C, react at low temperature for 30min, and slowly warm up to room temperature for 0.5 , cooled to -78°C again and added 300mL of compound M-1 (3.3g, 10mmol) toluene solution dropwise, slowly rose to room temperature and reacted for 10h, added ammonium chloride aqueous solution to quench the reaction, extracted with dichloromethane, and rotary evaporated Remove the solvent and dry to obtain the crude product of compound M-3;
[0062] Under the protect...
Embodiment 3
[0065] This embodiment provides a boron-containing compound, which has the structure shown in the following formula C-2:
[0066]
[0067] The difference between the preparation steps of the boron-containing compound shown in formula C-2 and the preparation step of the boron-containing compound shown in C-1 provided in Example 1 is only:
[0068] Compound M-2 in Example 1 was replaced by a compound represented by the following formula A-1. Yield 69%,
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com