Ruthenium complex fluorescence probe, preparation method and application
A technology of fluorescent probes and ruthenium complexes, which is applied in the fields of ruthenium organic compounds, fluorescence/phosphorescence, platinum group organic compounds, etc., can solve the problems of undeveloped and designed ruthenium anti-tumor drugs, unfavorable naked eye detection, poor selectivity, etc. Achieve the effect of excellent selectivity, good selectivity and simple material structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The present invention comprises the steps:
[0033] (1)Cis-[Ru(bpy) 2 Cl 2 ].2H 2 o
[0034] Weigh RuCl at a molar ratio of 1:1:2 3 ·3H 2 O. Lithium chloride monohydrate and auxiliary ligand 2,2'-bipyridyl, add DMF to dissolve to obtain a solution, heat under reflux at 140°C to obtain a solid-liquid mixture, add acetone after cooling to room temperature, and store at -4°C for 15 to 30 hours. Suction filtration to obtain purple black crystals, set aside;
[0035] (2) O-phenanthroline 5,6-dione
[0036] Add 4g of o-phenanthroline and 4g of potassium bromide, add a mixed solution of 40ml of ice-cold concentrated sulfuric acid and 20ml of concentrated nitric acid under magnetic stirring, add the mixed acid within 20 minutes, keep at 80-85°C, and reflux for 3h. After the reaction was complete, let the bromine escape, add ice to the completely cooled orange-yellow reaction product, neutralize it with NaOH until the pH was less than 7, extract the neutralized mixture di...
Embodiment 2
[0046] Adopt the preparation method similar to embodiment 1, specifically comprise the following steps:
[0047] (1)Cis-[Ru(phen) 2 Cl 2 ].2H 2 o
[0048] Weigh RuCl at a molar ratio of 1:1:2 3 ·3H 2 O. Lithium chloride monohydrate and the auxiliary ligand o-phenanthroline were dissolved in DMF to obtain a solution, heated at reflux at 140°C to obtain a solid-liquid mixture, cooled to room temperature, added acetone, stored at -4°C for 15 to 30 hours, and obtained by suction filtration Purple black crystal, for use;
[0049] (2) O-phenanthroline 5,6-dione
[0050] Add 4g of o-phenanthroline and 4g of potassium bromide, add a mixed solution of 40ml of ice-cold concentrated sulfuric acid and 20ml of concentrated nitric acid under magnetic stirring, add the mixed acid within 20 minutes, keep at 80-85°C, and reflux for 3h. After the reaction was complete, let the bromine escape, add ice to the completely cooled orange-yellow reaction product, neutralize it with NaOH until t...
Embodiment 3
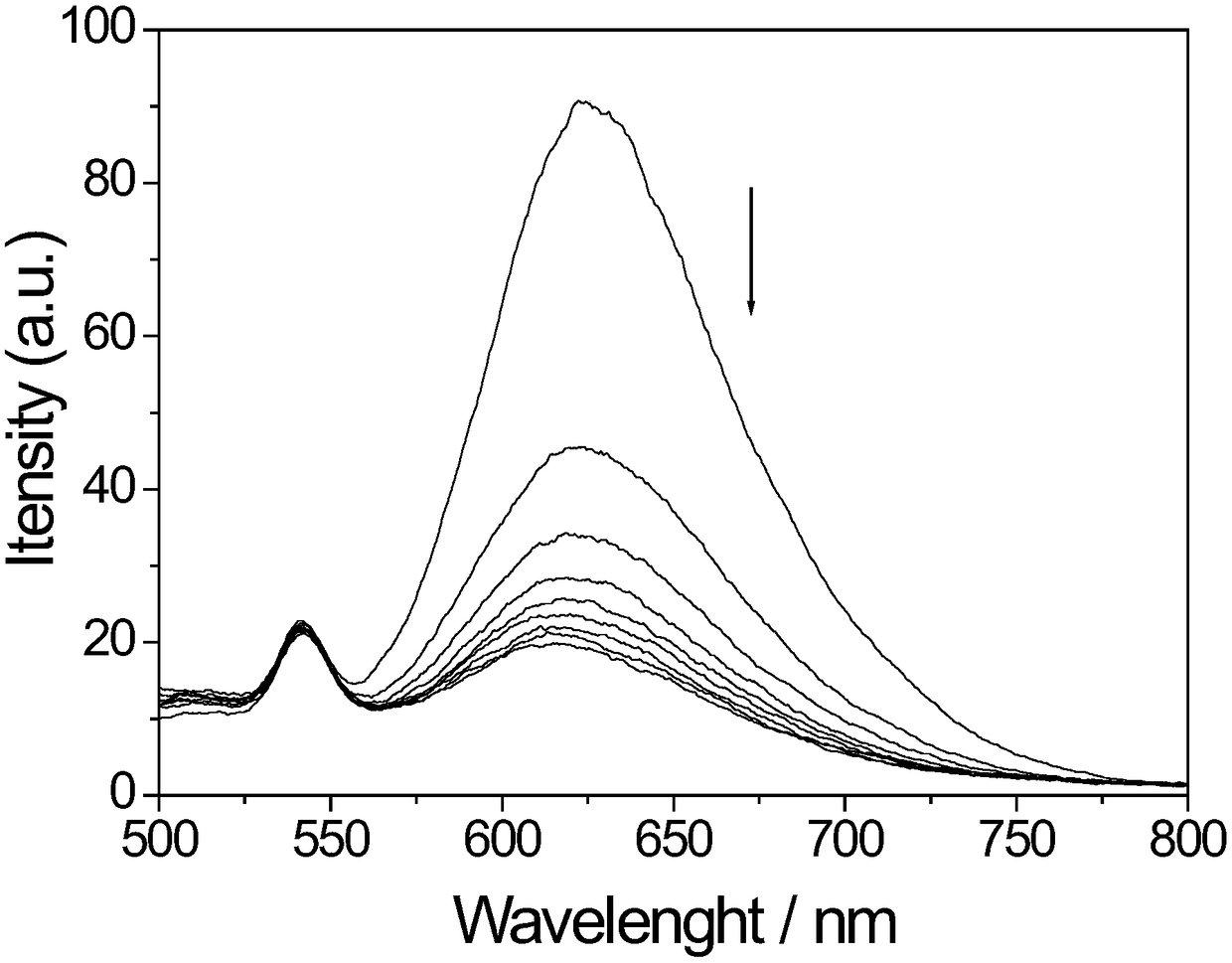
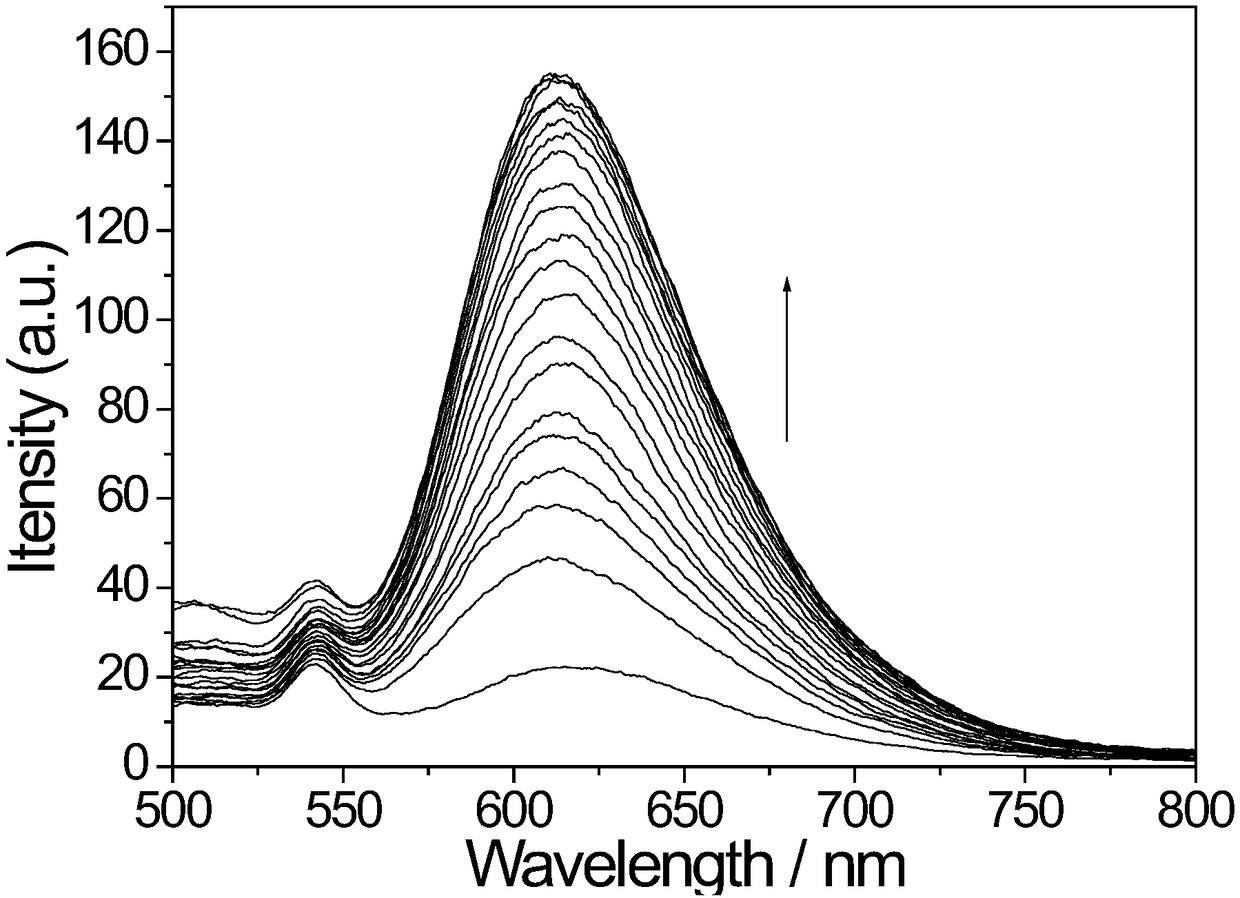
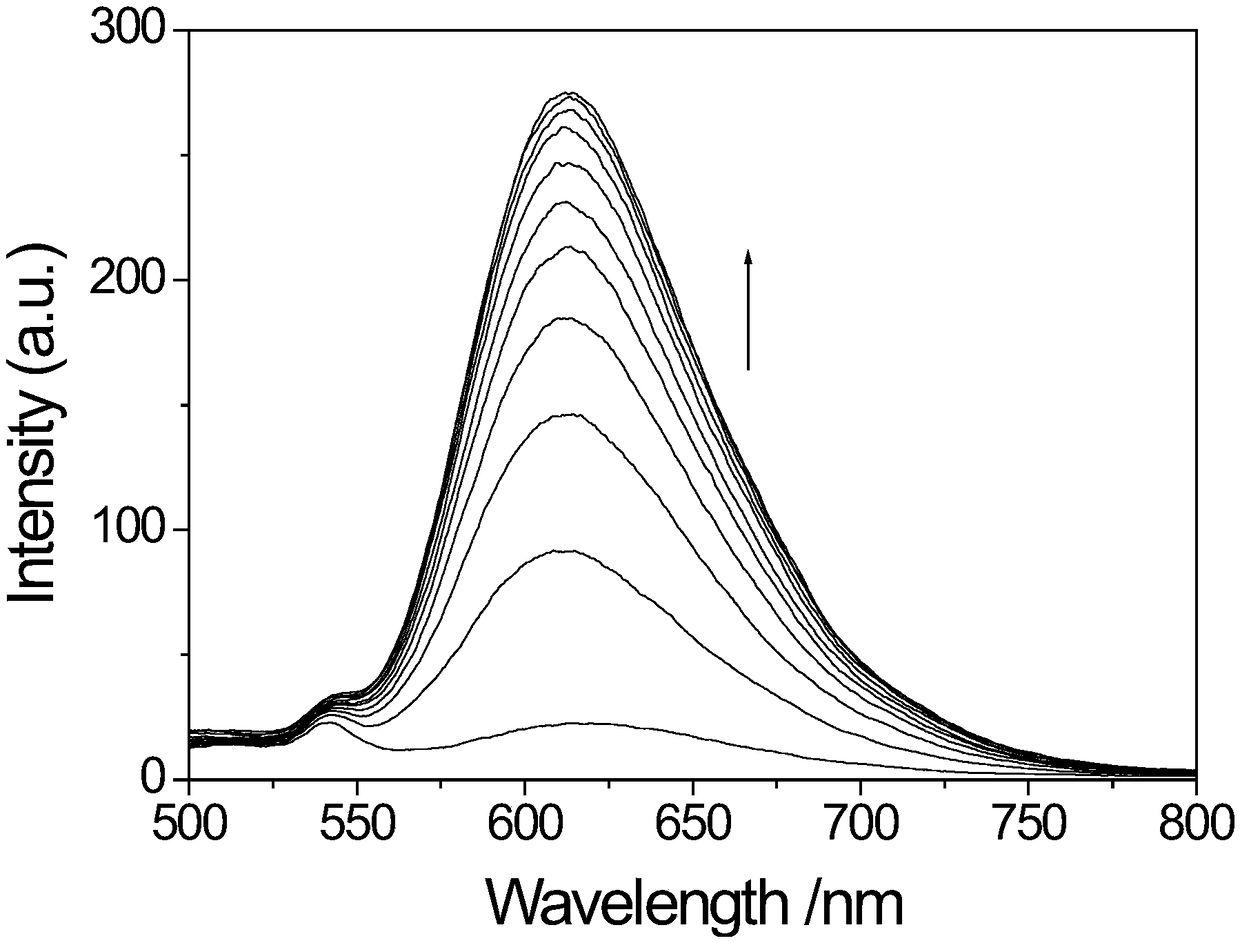
[0058] Applications of Probe Molecules
[0059] 1. Tris-HCl buffer:
[0060] Buffer A: 10mM Tris, 100mM KCl, pH=7.0;
[0061] General preparation method: Accurately weigh 0.303g Tris salt, 1.865g KCl l, dissolve completely with 60mL sterilized triple distilled water, slowly adjust the pH value to 7.0 with dilute hydrochloric acid, transfer to a 250ml volumetric flask, constant volume with triple distilled water, mix well Backup.
[0062] 2. Preparation of complex solution:
[0063] Accurately weigh 2 to 3 mg of the complex (depending on the molecular weight of the complex, the expected concentration of the ruthenium complex prepared in this paper is 200 μM, the volume is 10 mL, and the preparation container is a 10 mL volumetric flask, so the theoretical value to be weighed is: molecular weight / 1000*2mg), first dissolved in 50-100μL DMSO, then distilled to 10mL with pure water to obtain a 200μM stock solution of the complex.
[0064] 3. There are two types of double-stran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com