Method for efficient enzyme catalytic synthesis of sanguinarine and chelerythrine
A technology for chelerythrine and sanguinarine, which is applied in the field of enzyme catalytic synthesis of sanguinarine and chelerythrine, can solve the problems of limited improvement potential, limited sources, low efficiency, etc., so as to improve the catalytic efficiency of enzymes, Achieve the effect of comprehensive utilization and optimization of fermentation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] In order to better illustrate the content of the invention, the present invention will be further verified by specific examples below. It is specially stated here that the embodiments are only for describing the present invention more directly, they are only a part of the present invention, and cannot constitute any limitation to the present invention.
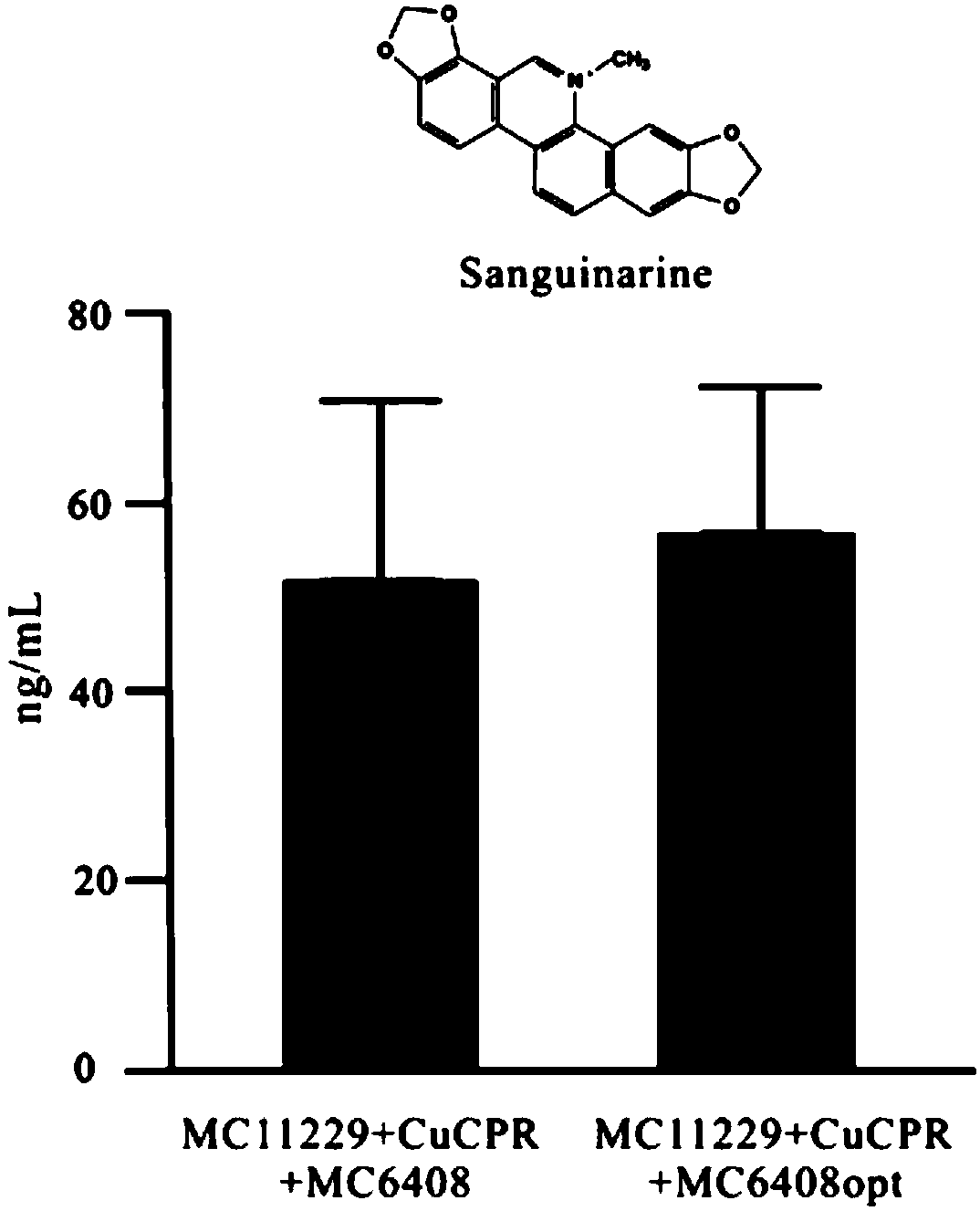
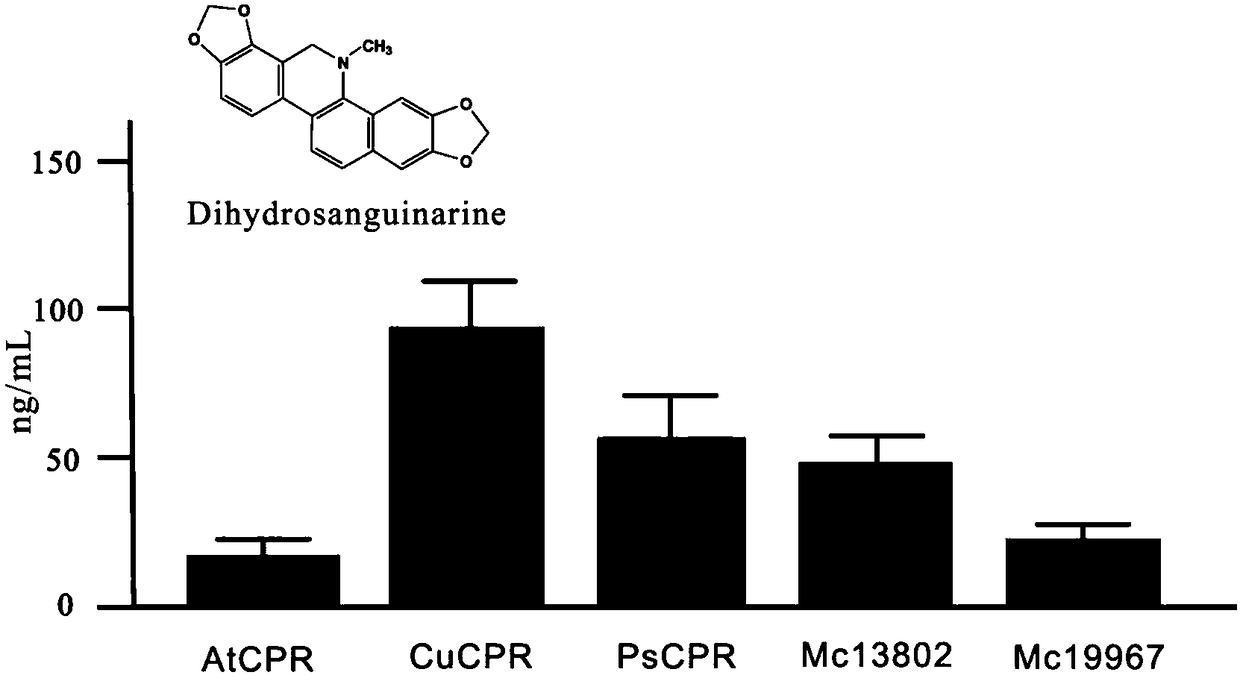
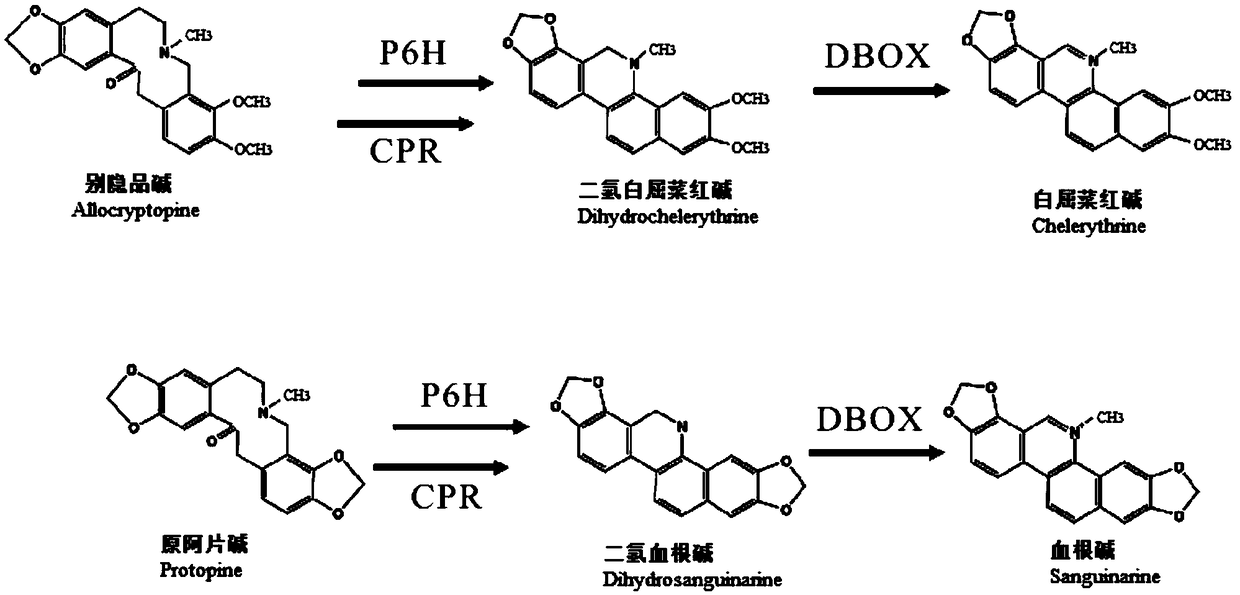
[0052] as attached figure 1 The synthetic pathway of sanguinarine and chelerythrine in Boluohui shows that protropine-6-hydroxylase (P6H) is involved in the biogenesis of sanguinarine (SAN) and chelerythrine (CHE). Synthetic steps, which can catalyze allocryptine (ALL) to generate dihydrochelerythrine (DHCHE) and catalyze proopiate (PRO) to generate dihydrosanginarine (DHSAN). Since P6H belongs to cytochrome P450 oxidoreductase, as a monooxygenase, it needs to be co-expressed with cytochrome P450 reductase (CPR) to play a role in protein catalysis, and CPR plays the role of transferring electrons in the reaction. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com