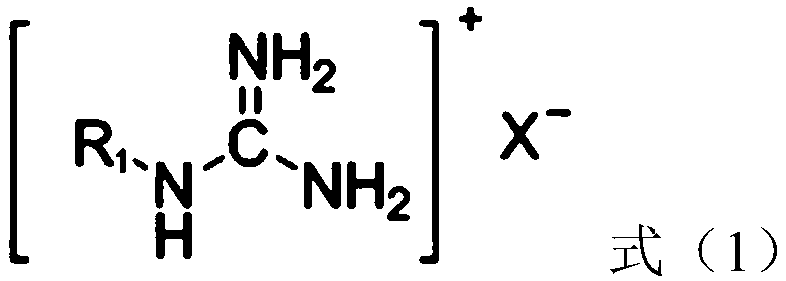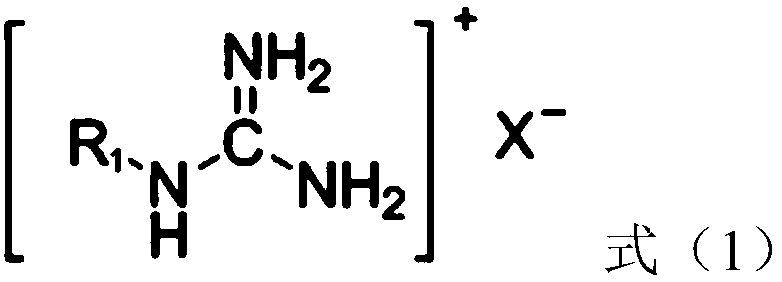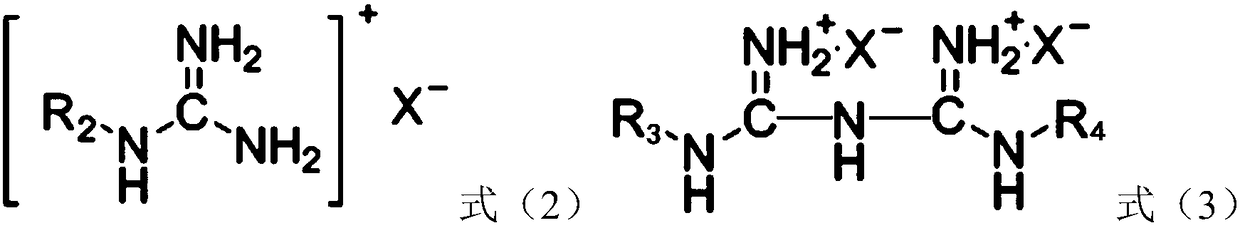Reagent composition for bleaching of textiles, and textile bleaching method
A composition and textile technology, applied in textiles and papermaking, etc., can solve problems such as poor water solubility and harmful by-products, and achieve the effects of avoiding environmental problems, easy handling, and improving bleaching performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) 2.75g sucrose ester, 2.75g sodium edetate and 3g sodium bicarbonate were dissolved in 86.2g water to prepare bleaching aid;
[0053] (2) Immerse the polylactic acid filament / viscose filament interwoven fabric (a polylactic acid fabric) in the bleaching aid solution with a bath ratio of 1:50. Under ultrasonic stirring, the temperature was raised to 55°C at a rate of 3.5°C / min, and hydrogen peroxide, the compound with the structure shown in formula (1-1) and the compound with the structure shown in formula (2-1) were added. The mass of hydrogen peroxide, the compound of structure shown in formula (1-1) and the compound of structure shown in formula (2-1) are respectively 3.3g, 1.67g and 0.33g;
[0054] (3) Bleaching was carried out at 55° C. for 45 minutes under ultrasonic stirring, and the polylactic acid filament / viscose filament interwoven fabric was taken out, washed with water and dried to obtain bleached fabric A1.
[0055]
Embodiment 2
[0057] (1) 5g sorbitan ester, 0.5g sodium silicate and 1g potassium dihydrogen phosphate are dissolved in 83.5g water to prepare bleaching aid;
[0058] (2) Soybean protein knitted fabrics are immersed in the bleaching aid solution with a bath ratio of 1:50. Under ultrasonic stirring, the temperature was raised to 60°C at a rate of 2°C / min, and sodium perborate, the compound with the structure shown in formula (1-2) and the compound with the structure shown in formula (2-2) were added, wherein, over The quality of sodium borate, the compound of structure shown in formula (1-2) and the compound of structure shown in formula (2-2) are respectively 6g, 3.64g and 0.36g;
[0059] (3) Bleaching was carried out at 60° C. for 60 minutes under ultrasonic stirring, and the soybean protein knitted fabric was taken out, washed with water and dried to obtain bleached fabric A2.
[0060]
Embodiment 3
[0062] (1) Dissolve 0.5g of polyoxyethylene amine, 5g of sodium pyrophosphate and 5g of sodium acetate in 88.5g of water to prepare a bleaching aid;
[0063] (2) The milk protein / modal knitted fabric (a kind of milk protein fabric) is immersed in the bleaching aid, and the bath ratio is 1:50. Under ultrasonic stirring, the temperature was raised to 50°C at a rate of 5°C / min, and percarbamide, the compound of the structure shown in formula (1-3) and the compound of the structure shown in formula (2-3) were added, wherein, The mass of carbonamide, the compound of structure shown in formula (1-3) and the compound of structure shown in formula (2-3) are respectively 0.6g, 0.307g and 0.093g;
[0064] (3) Bleaching was carried out at 50° C. for 30 minutes under ultrasonic stirring, and the milk protein / modal knitted fabric was taken out, washed with water and dried to obtain bleached fabric A3.
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com