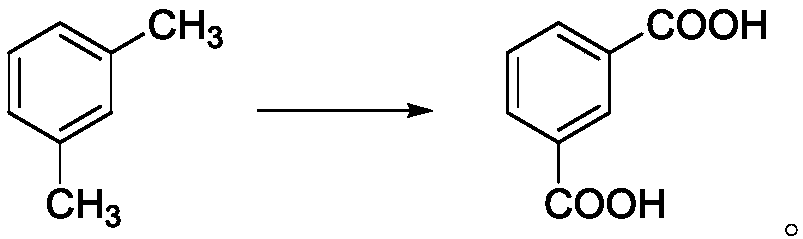
Process for preparing m-phthalic acid
A technology for the preparation of isophthalic acid and its preparation process, applied in the field of preparation of isophthalic acid, can solve the problems of high energy consumption and cost, high greenhouse gas emissions, high reaction temperature, etc., and achieve reduction of production cost, energy consumption, and energy consumption The effect of low, high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the mensuration of isophthalic acid yield and purity
[0051] Isophthalic acid yield and purity are analyzed and tested by high performance liquid chromatography, and the specific instruments and test conditions are as follows:
[0052] The HPLC detection method adopts the American Agilent 1120 high performance liquid chromatograph, automatic sample injector, Agilent 1120 UV / Vis detector, Empower 2 data processing system.
[0053] Chromatographic conditions:
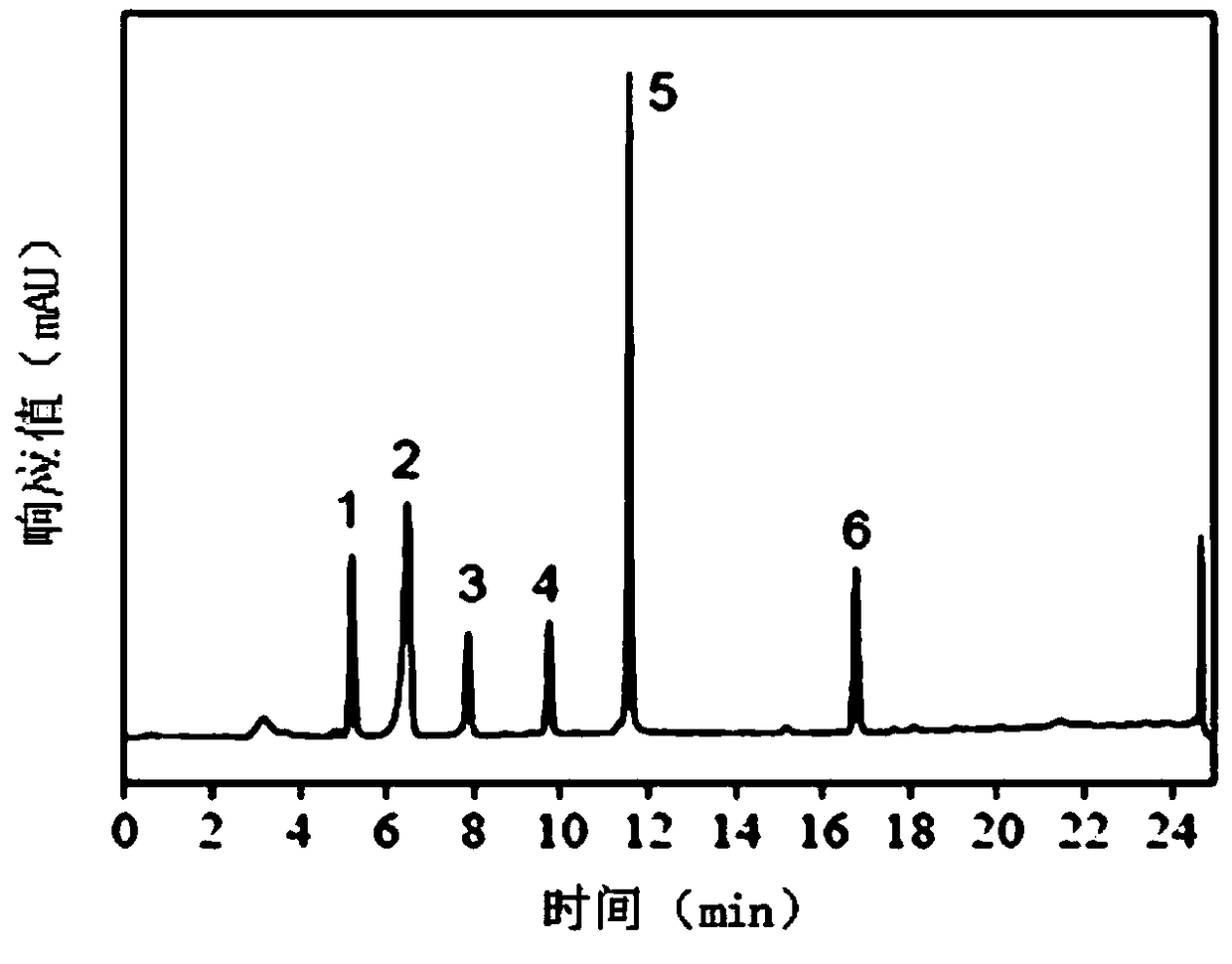
[0054] Chromatographic column: Agilent TC-C18 chromatographic column (4.6×250mm, 5μm); mobile phase A is 100% acetonitrile, mobile phase B is 17% methanol + 83% water; gradient elution conditions: A from 30% in 0 to 20min to 100%, B from 100% to 30% in 20~25min, B from 70% to 0% in 0~20min, B from 0% to 70% in 20~25min; flow rate: 1mL / min; column temperature: 30 ℃; detection wavelength: 260nm; injection volume: 20μL. Under this condition, the retention time of the main peak of isophthalic acid is ...
Embodiment 2
[0058] Embodiment 2: the preparation of isophthalic acid crude product
[0059] Add 10mL of m-xylene, 24mL of acetonitrile, and 16mL of water into a 100mL round-bottomed flask, keep the pH value at 4-4.5, stir with a six-blade impeller, and bubble ozone into the solution at a flow rate of 20ml / min to keep The pressure is normal pressure, the temperature is room temperature, the ultraviolet light is continuously irradiated for 20 hours, and the power of the ultraviolet lamp is 100W (200mW / cm 2 , 310nm), after the reaction finishes, the m-xylene conversion rate is 98%, and the isophthalic acid yield is 96%, and the m-toluic acid content is 2%, and the 3-CBA content is 160ppm (10 -6 g / g).
Embodiment 3
[0060] Embodiment 3: the preparation of isophthalic acid crude product
[0061] Add 25mL of m-xylene, 15mL of acetonitrile, and 10mL of water into a 100mL round-bottomed flask, keep the pH value at 4 to 4.5, stir with a six-blade impeller, and bubble ozone into the solution. The flow rate of ozone is 9mL / min, and keep The pressure is normal pressure, the temperature is 25°C, the ultraviolet light is continuously irradiated for 20 hours, and the power of the ultraviolet lamp is 100W (200mW / cm 2 , 310nm). After the reaction, the remaining amount of m-xylene was 0.006 mol, the content of isophthalic acid was 0.131 mol, the conversion rate of m-xylene was 97%, and the yield of isophthalic acid was 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com