Method for selectively synthesizing hexafluoropropylene oxide oligomer
A hexafluoropropylene oxide, selective technology, applied in the field of selective synthesis of hexafluoropropylene oxide oligomers, can solve the problems of low reaction temperature, low trimer selectivity, limited use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
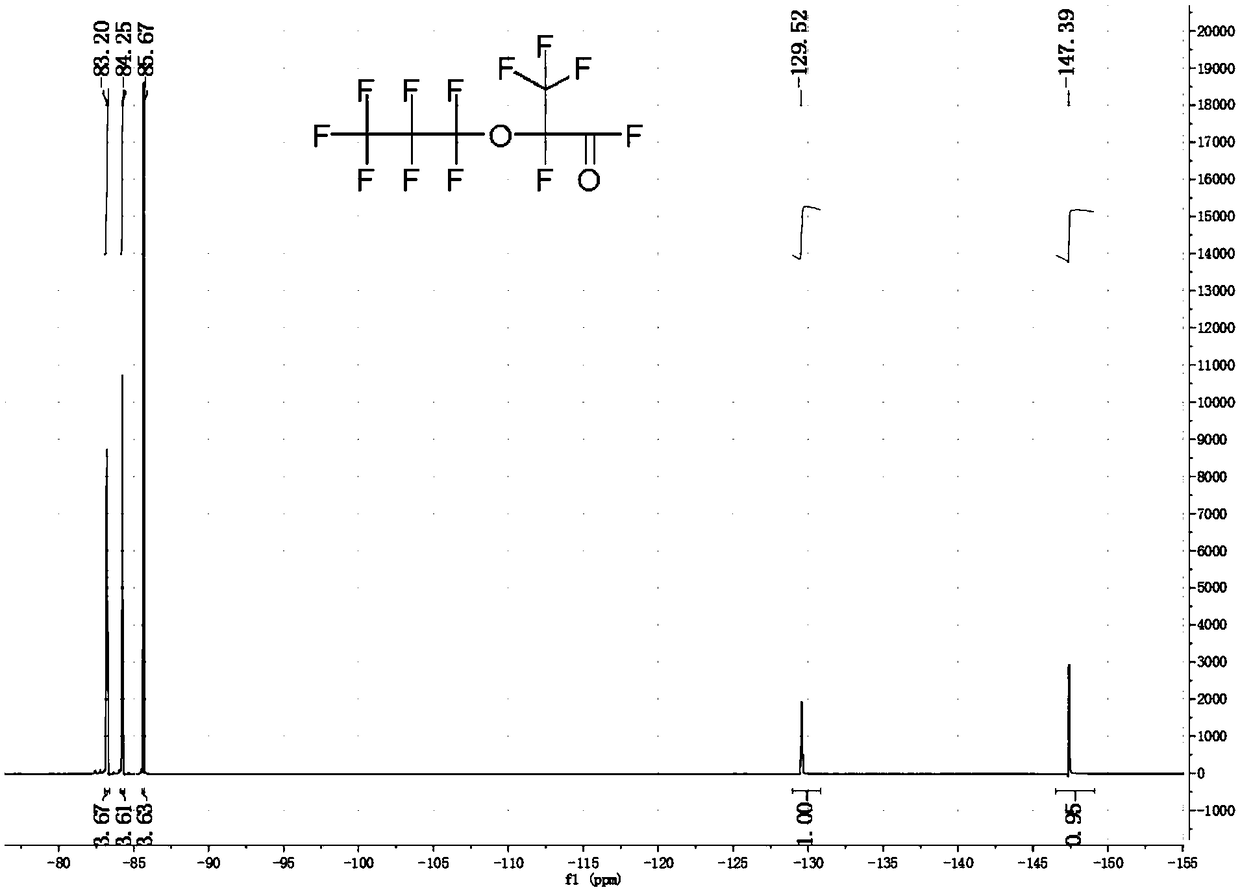
[0047] Weigh 2.33g (0.04mol) of anhydrous potassium fluoride, 17.8g of tetraethylene glycol dimethyl ether and 7.27g (0.04mol) of P, P-diisopropoxyphosphonamide, and add them to a 500mL 316L In a reaction kettle, stir and cool to 0°C under a sealed environment. Open the feed valve to feed 200g (1.2mol) of hexafluoropropylene oxide gas into the reactor, and stabilize the reaction temperature at 40±5°C by regulating the temperature of the cooling liquid and controlling the intake rate; To room temperature, discharge, and liquid separation to obtain 192.85 g of crude product of hexafluoropropylene dimer in the lower layer of colorless transparent oil phase, the content of dimer is 86.54%, and the content of trimer and tetramer is 13.46%.
[0048] The product in this example is carried out structural identification (19F-NMR), the result is as follows figure 1 as shown, figure 1 It is the fluorine spectrum of the product in Example 1 of the present invention. Depend on figure 1...
Embodiment 2
[0050] Take by weighing 11.65g (0.2mol) anhydrous potassium fluoride, 89.00g tetraethylene glycol dimethyl ether and 39.96g (0.2mol) P-isopropoxy-P-phenylphosphonamide, add to In a 2000mL 316L reaction kettle, stir in a sealed environment and cool to -10°C. Open the feed valve to feed 1000g (6.0mol) of hexafluoropropylene oxide gas into the reactor, and stabilize the reaction temperature at 50±5°C by regulating the temperature of the cooling liquid and controlling the intake velocity; to room temperature, discharge, and liquid separation to obtain 993.35 g of crude product of hexafluoropropylene dimer in the lower colorless transparent oil phase, the content of dimer is 85.94%, and the content of trimer and tetramer is 14.06%.
Embodiment 3
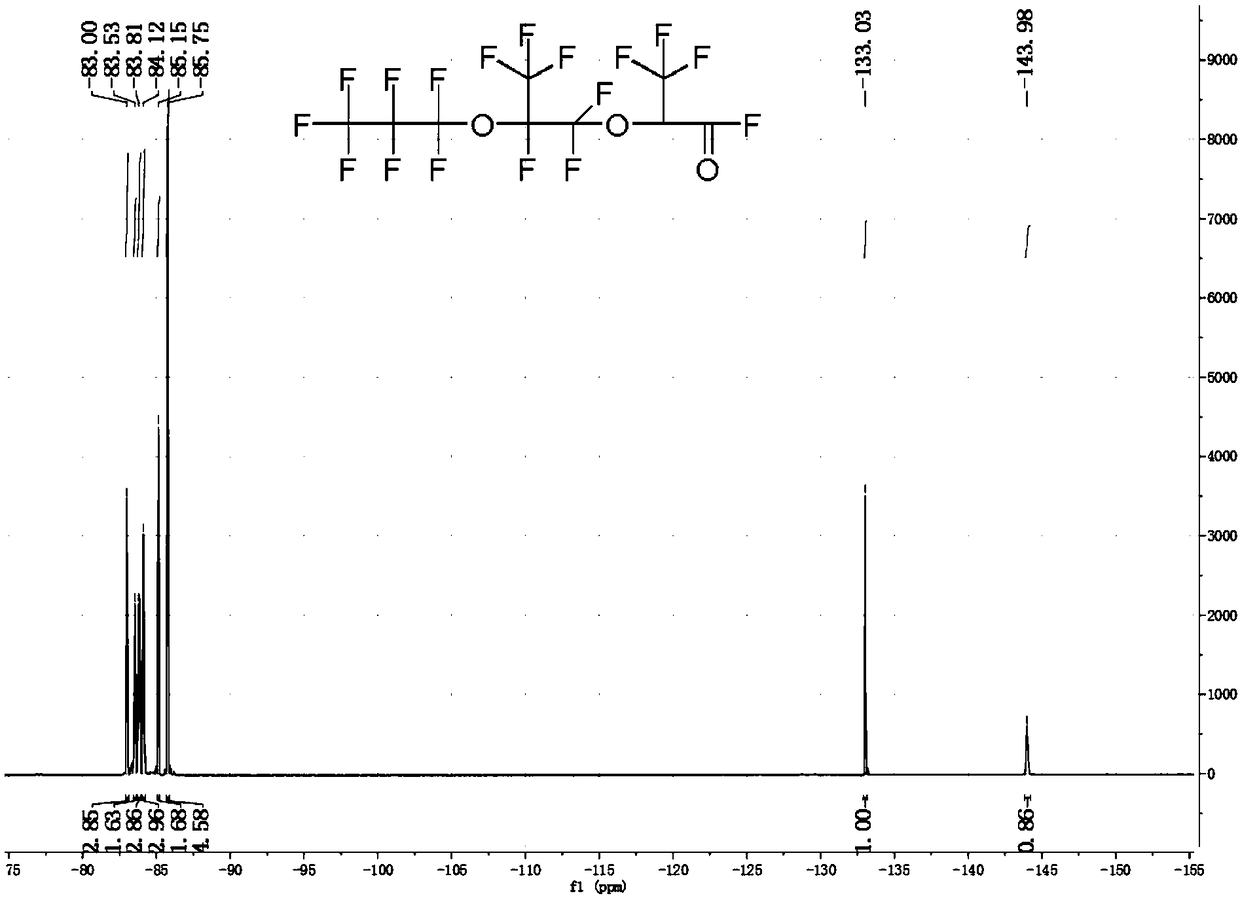
[0052] Weigh 2.07g (0.02mol) of anhydrous zinc fluoride, 17.8g of tetraethylene glycol dimethyl ether and 7.27g (0.04mol) of P, P-diisopropoxyphosphonamide, and add it to a 500mL 316L In a reaction kettle, stir and cool to 0°C under a sealed environment. Open the feed valve to feed 200g (1.2mol) of hexafluoropropylene oxide gas into the reactor, and stabilize the reaction temperature at 40±5°C by regulating the temperature of the cooling liquid and controlling the intake rate; To room temperature, discharge, liquid separation to obtain 195.09 g of crude hexafluoropropylene trimer in the lower layer of colorless transparent oil phase, the content of trimer is 84.59%, and the content of dimer and tetramer is 15.41%.
[0053] The product in this example is carried out structural identification (19F-NMR), the result is as follows figure 2 as shown, figure 1 It is the fluorine spectrum of the product in Example 3 of the present invention. Depend on figure 2 It can be seen tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com