Liquid-phase CO2 methanation catalyst, preparation method and application of catalyst
一种甲烷化催化剂、CO2的技术,应用在物理/化学过程催化剂、催化反应、化学仪器和方法等方向,能够解决催化剂强度下降、消耗较大能量、催化剂层侵蚀等问题,达到防止聚集、实现回收和循环利用、避免高温易脱落的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
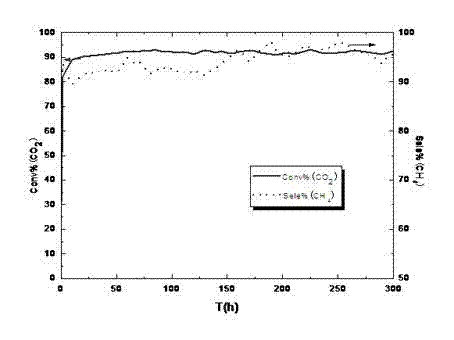
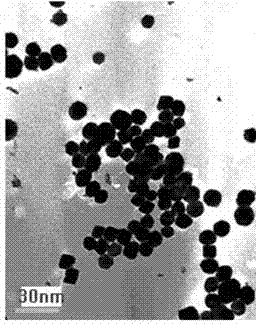
[0028] At room temperature, nickel nitrate, magnesium nitrate and 1-16 alkyl-3-methylimidazole hydrochloride ionic liquid are mixed and dispersed in water at a molar ratio of 1:0.2:20 to obtain a mixed system solution. The concentration of nickel nitrate in the system solution is 2.79×10 -4 mol / L, then add ammonia water to adjust the pH value of the system to 9.0, continue to stir and react for 3h, then transfer the reaction solution to the reactor at 150 o C, H at 3.0MPa 2 Reduce for 2h, finally centrifuge, filter, wash with water, wash with ethanol, and dry. After TEM test, see figure 1 . by figure 1 It can be seen that the liquid CO 2 The metal active component nanoparticles in the methanation catalyst have an average particle size of 11.8nm, a narrow particle size distribution and a uniform distribution.
[0029] Use the above catalyst for CO 2 The methanation reaction is specifically: add an appropriate amount of the above catalyst into the reaction kettle, and then add an a...
Embodiment 2
[0031] At room temperature, nickel acetate, lanthanum nitrate and 1-18 alkyl-3-methylimidazole hexafluorophosphate ionic liquid are mixed and dispersed in the liquid medium ethanol at a molar ratio of 1:0.15:20 to obtain a mixed system solution. The amount and concentration of the nickel acetate substance is 3.61×10 -4 mol / L, then add triethylamine to adjust the pH value of the system to 8.0, continue to stir and react for 2h, then transfer the reaction solution to the reactor at 100 o C, pass H under 3.0MPa pressure 2 It is reduced for 1.5h, and finally centrifuged, and then filtered, washed with water, washed with ethanol, and dried.
[0032] Use the above catalyst for CO 2 The methanation reaction is specifically as follows: add an appropriate amount of the above catalyst into the reactor, and then add an appropriate amount of solvent water, and control the concentration of the catalyst in the reaction system to 0.005mol / L, according to nH 2 : NCO 2 =4, access to CO 2 And H 2 , ...
Embodiment 3
[0034] At room temperature, nickel nitrate, cerium nitrate and amphiphilic ionic liquid N-dodecylpyridine tetrafluoroborate (C 12 PyBF 4 ) The molar ratio is 1:0.15:18, mixed and dispersed in the liquid medium acetonitrile to obtain a mixed system solution. The amount and concentration of nickel nitrate in the mixed system is 1.32×10 -3 mol / L, then add diisopropyl tert-butylamine to adjust the pH value of the system to 10.0, continue to stir and react for 3h, then transfer the reaction liquid to the reactor at 150 o C, pass H under 3.0MPa pressure 2 It is reduced for 1.5h, finally centrifuged, filtered, washed, and dried.
[0035] Use the above catalyst for CO 2 The methanation reaction is specifically: add an appropriate amount of the above catalyst into the reaction kettle, and then add an appropriate amount of solvent acetonitrile, control the concentration of the catalyst in the reaction system to 0.005mol / L, according to nH 2 : NCO 2 =4, access to CO 2 And H 2 , And then heate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com