A kind of method of selective synthesis hexafluoropropylene oxide oligomer
A hexafluoropropylene oxide and selective technology, which is applied in the field of selective synthesis of hexafluoropropylene oxide oligomers, can solve the problems of low reaction temperature, long reaction time, restricted use and the like, and achieves environmentally friendly and low-cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
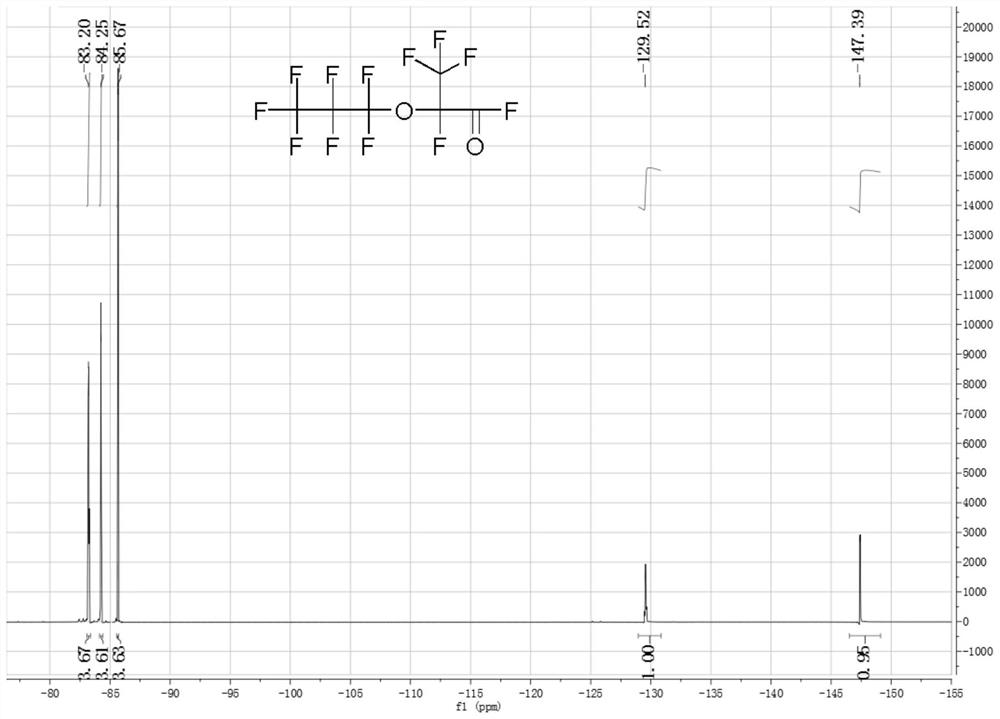
[0047] Weigh 2.33g (0.04mol) of anhydrous potassium fluoride, 17.8g of tetraethylene glycol dimethyl ether and 7.27g (0.04mol) of P, P-diisopropoxyphosphonamide, and add them to a 500mL 316L In a reaction kettle, stir and cool to 0°C under a sealed environment. Open the feed valve to feed 200g (1.2mol) of hexafluoropropylene oxide gas into the reactor, and stabilize the reaction temperature at 40±5°C by regulating the temperature of the cooling liquid and controlling the intake rate; To room temperature, discharge, and liquid separation to obtain 192.85 g of crude product of hexafluoropropylene dimer in the lower layer of colorless transparent oil phase, the content of dimer is 86.54%, and the content of trimer and tetramer is 13.46%.
[0048] The product in this example is carried out structural identification (19F-NMR), the result is as follows figure 1 as shown, figure 1 It is the fluorine spectrum of the product in Example 1 of the present invention. Depend on figure 1...
Embodiment 2
[0050] Take by weighing 11.65g (0.2mol) anhydrous potassium fluoride, 89.00g tetraethylene glycol dimethyl ether and 39.96g (0.2mol) P-isopropoxy-P-phenylphosphonamide, add to In a 2000mL 316L reaction kettle, stir in a sealed environment and cool to -10°C. Open the feed valve to feed 1000g (6.0mol) of hexafluoropropylene oxide gas into the reactor, and stabilize the reaction temperature at 50±5°C by regulating the temperature of the cooling liquid and controlling the intake velocity; to room temperature, discharge, and liquid separation to obtain 993.35 g of crude product of hexafluoropropylene dimer in the lower colorless transparent oil phase, the content of dimer is 85.94%, and the content of trimer and tetramer is 14.06%.
Embodiment 3
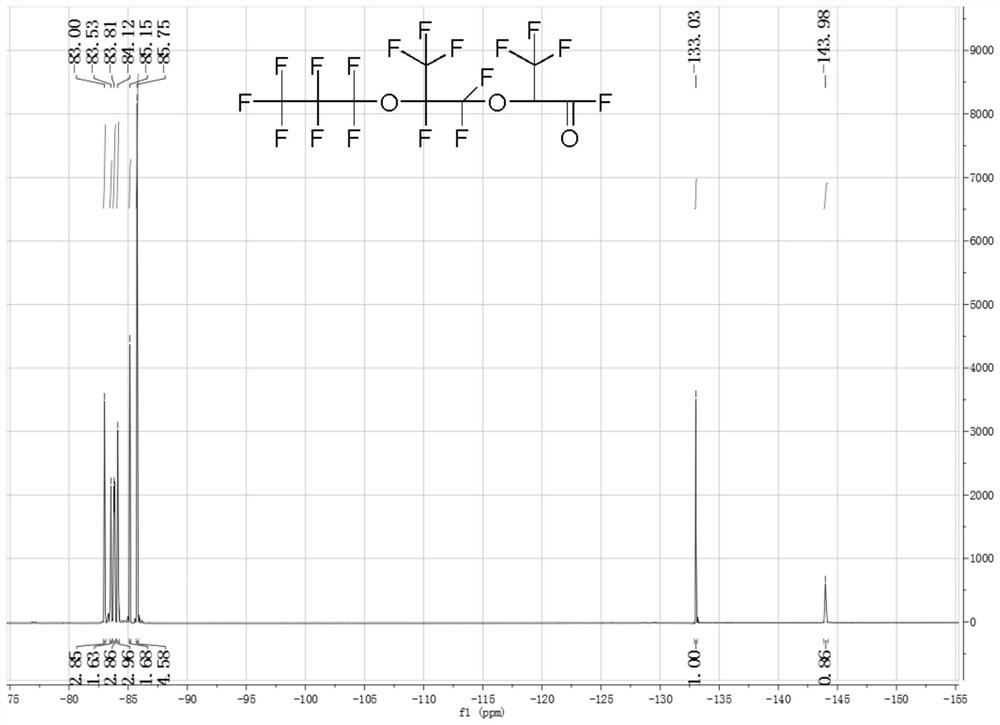
[0052] Weigh 2.07g (0.02mol) of anhydrous zinc fluoride, 17.8g of tetraethylene glycol dimethyl ether and 7.27g (0.04mol) of P, P-diisopropoxyphosphonamide, and add it to a 500mL 316L In a reaction kettle, stir and cool to 0°C under a sealed environment. Open the feed valve to feed 200g (1.2mol) of hexafluoropropylene oxide gas into the reactor, and stabilize the reaction temperature at 40±5°C by regulating the temperature of the cooling liquid and controlling the intake rate; To room temperature, discharge, liquid separation to obtain 195.09 g of crude hexafluoropropylene trimer in the lower layer of colorless transparent oil phase, the content of trimer is 84.59%, and the content of dimer and tetramer is 15.41%.
[0053] The product in this example is carried out structural identification (19F-NMR), the result is as follows figure 2 as shown, figure 2 It is the fluorine spectrum of the product in Example 3 of the present invention. Depend on figure 2 It can be seen th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com