Method for synthesizing 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and methyl isobutyl ketone, which is applied in the field of 5-hydroxymethylfurfural preparation, can solve the problems of difficult separation and purification of 5-HMF, achieve easy separation, simple post-treatment, and reduce production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
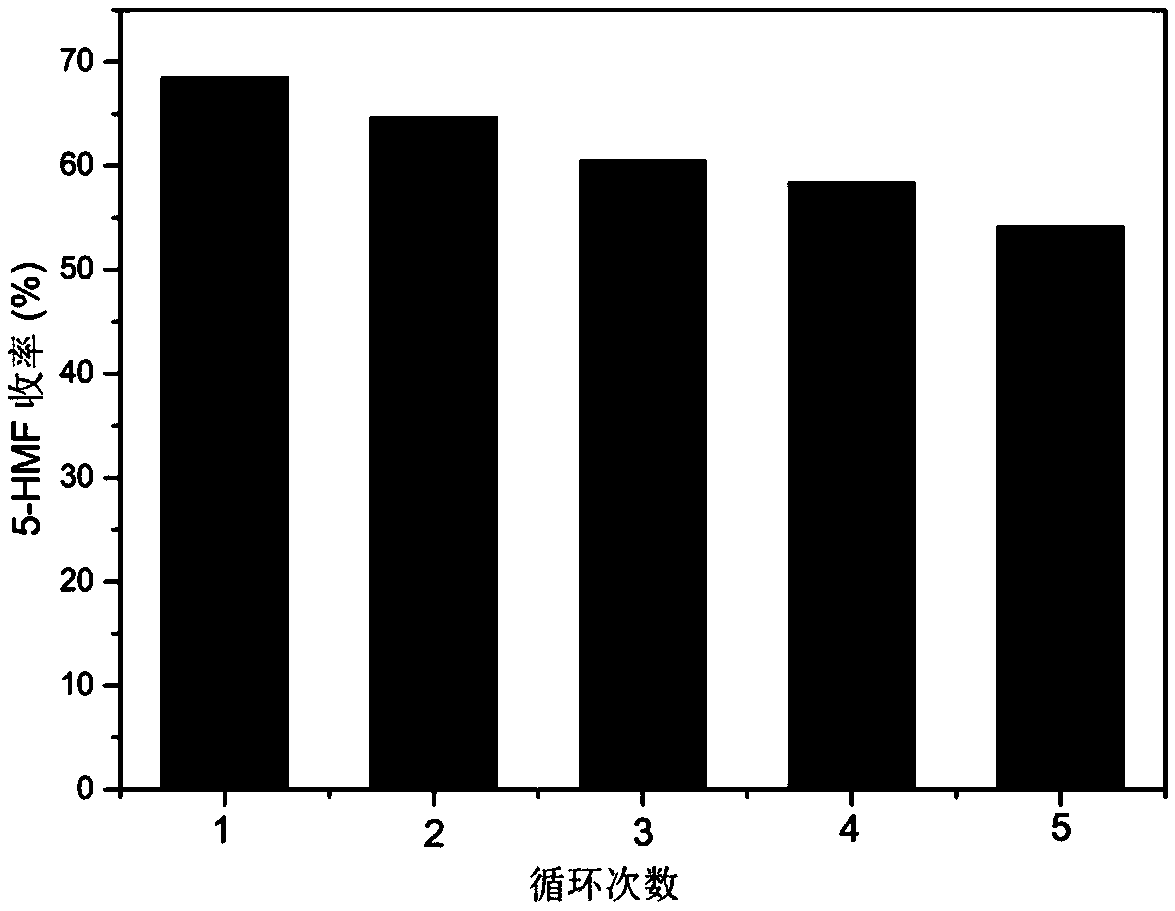
[0026] Add 50 mg of cellobiose and 25 mg of niobium-modified montmorillonite catalyst into a thick-walled pressure-resistant bottle (15 mL) reactor containing 5 mL of biphasic solvent at the same time. The biphasic solvent is saturated saline and methyl isobutyl Base ketone, the volume ratio is 1.5:3.5, and the thick-walled pressure-resistant bottle is placed in a constant temperature oil bath for reaction, and the reaction temperature is measured by a thermocouple inserted into the oil bath. The reactor has a built-in magnet, and the stirring rate is kept at 500rpm. After a certain reaction time, immediately transfer the reaction vial to an ice-water bath to quench the reaction. Then it is transferred to a suction filter funnel for suction filtration, and the solid catalyst obtained by suction filtration is weighed after washing and drying to ensure that the reuse reaction keeps the same reactant and catalyst ratio as the original reaction, and continues to be used in the nex...
Embodiment 2
[0028] Add 50 mg of cellobiose and 25 mg of niobium-modified montmorillonite catalyst into a thick-walled pressure-resistant bottle (15 mL) reactor containing 5 mL of biphasic solvent at the same time. The biphasic solvent is saturated saline and methyl isobutyl base ketone, the volume ratio is 1:4, and the thick-walled pressure-resistant bottle is placed in a constant temperature oil bath for reaction, and the reaction temperature is measured by a thermocouple inserted into the oil bath. The reactor has a built-in magnet, and the stirring rate is kept at 500rpm. After a certain reaction time, immediately transfer the reaction vial to an ice-water bath to quench the reaction. Then it is transferred to a suction filter funnel for suction filtration, and the solid catalyst obtained by suction filtration is weighed after washing and drying to ensure that the reuse reaction keeps the same reactant and catalyst ratio as the original reaction, and continues to be used in the next st...
Embodiment 3
[0030] Add 50 mg of cellobiose and 25 mg of niobium-modified montmorillonite catalyst into a thick-walled pressure-resistant bottle (15 mL) reactor containing 5 mL of biphasic solvent at the same time. The biphasic solvent is saturated saline and methyl isobutyl base ketone, the volume ratio is 2:3, and the thick-walled pressure-resistant bottle is placed in a constant temperature oil bath for reaction, and the reaction temperature is measured by a thermocouple inserted into the oil bath. The reactor has a built-in magnet, and the stirring rate is kept at 500rpm. After a certain reaction time, immediately transfer the reaction vial to an ice-water bath to quench the reaction. Then it is transferred to a suction filter funnel for suction filtration, and the solid catalyst obtained by suction filtration is weighed after washing and drying to ensure that the reuse reaction keeps the same reactant and catalyst ratio as the original reaction, and continues to be used in the next st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com