Injectable cartilage repairing hydrogel and preparation method thereof
A cartilage repair and hydrogel technology, applied in the field of biomedical engineering, can solve the problems of sudden release of growth factors, unsatisfactory treatment effect, inconvenient operation, etc., to achieve regeneration promotion, good cartilage induction repair ability, and convenient clinical operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
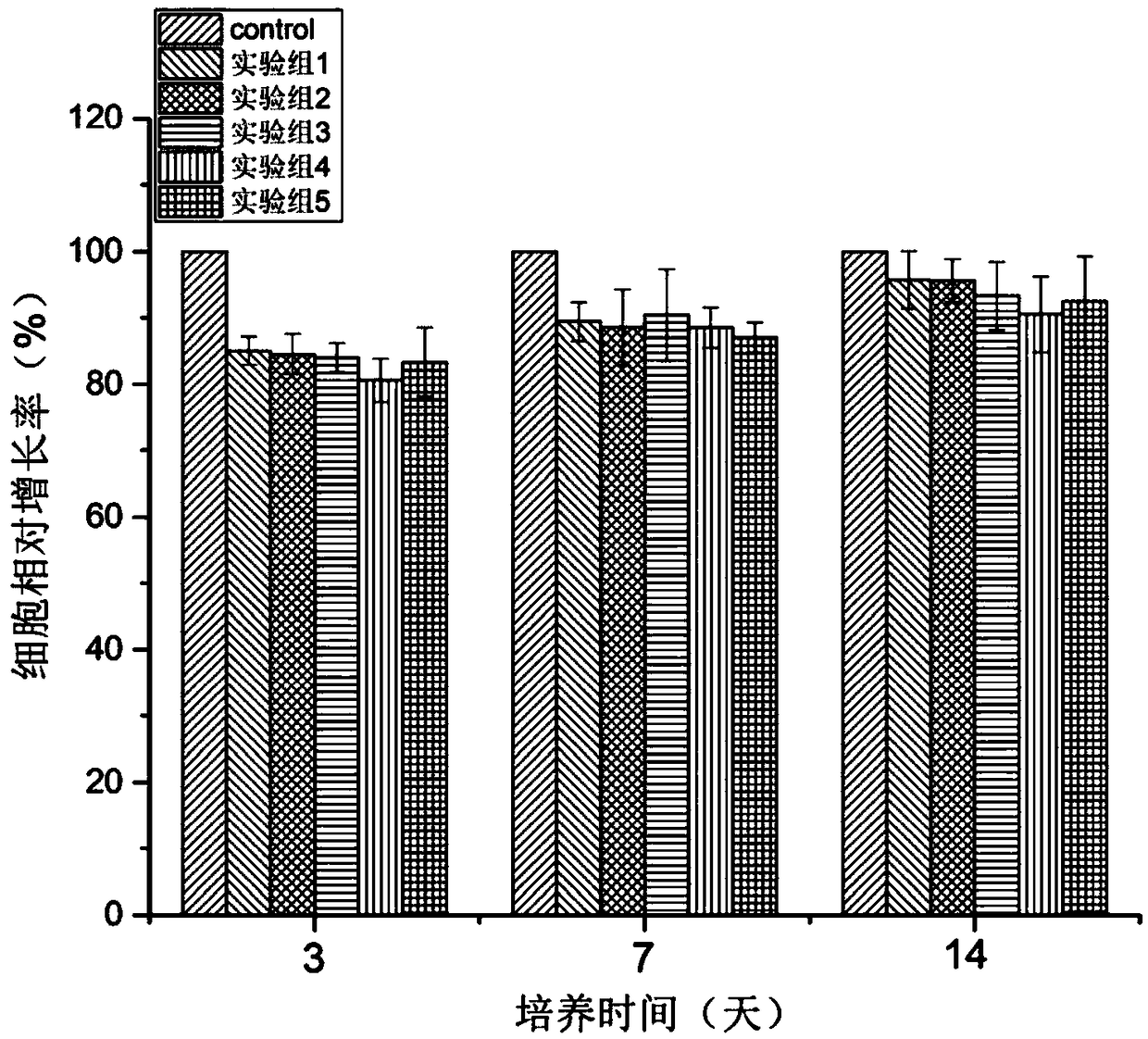
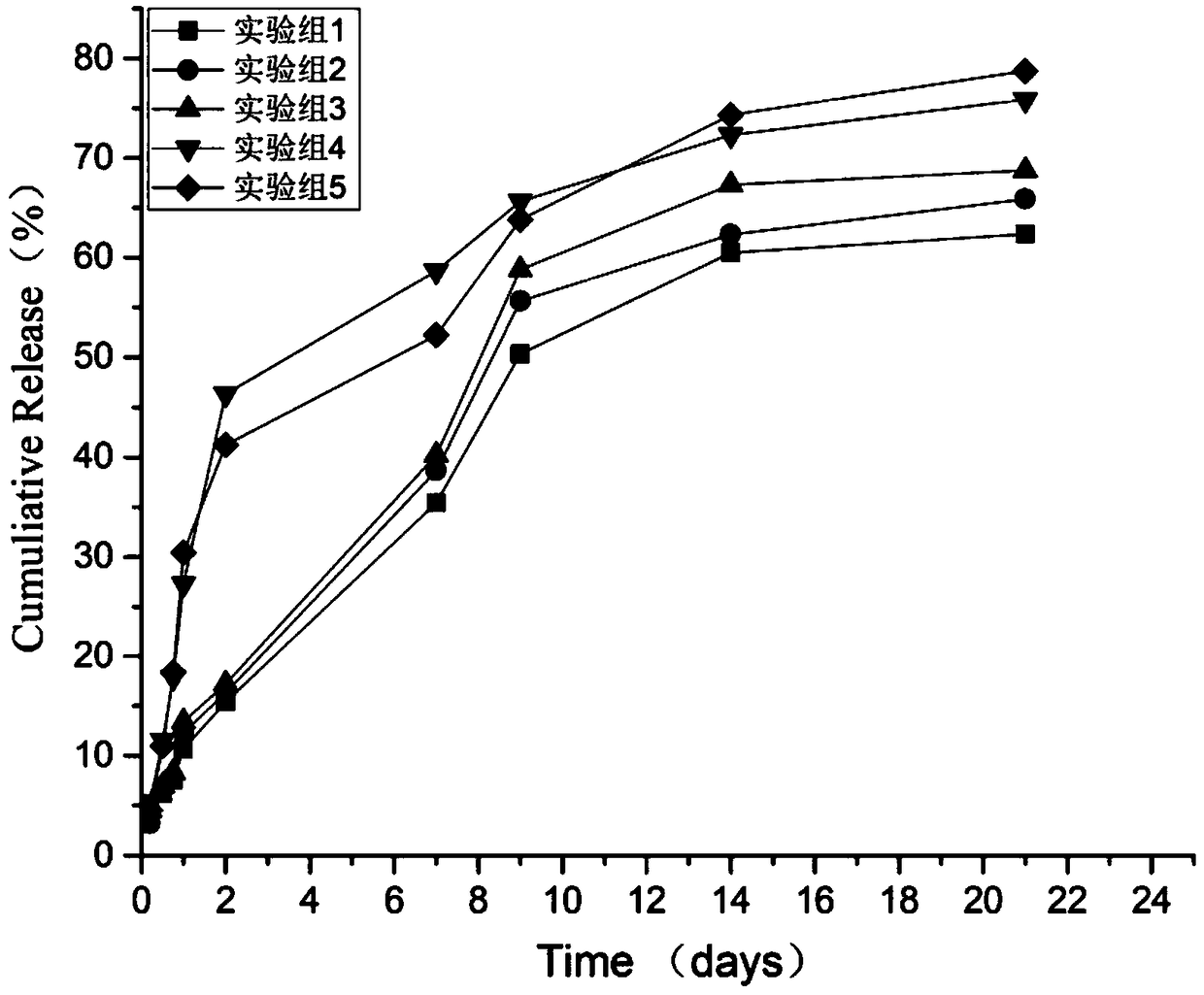
Image
Examples
Embodiment 1
[0030] An injectable hydrogel for cartilage repair, comprising the following components in parts by weight: 47.5 parts of aminated chondroitin sulfate, 47.5 parts of oxidized dextran, and 5 parts of sodium alginate microspheres encapsulating growth factors. The hydrogel is in the form of a liquid gel.
[0031] The preparation method of the present embodiment hydrogel is:
[0032] (1) Preparation of aminated chondroitin sulfate
[0033] Weigh chondroitin sulfate and dissolve it in distilled water to prepare a chondroitin sulfate solution with a concentration of 5 mg / mL, then adjust the pH of the chondroitin sulfate solution to 6.8 with a concentration of 0.1 mol / L NaOH solution and HCl solution, and then massage Mole ratio of chondroitin sulfate: adipic acid dihydrazide: 1-hydroxybenzotriazole: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride = 1:10:5 : 5, add adipic dihydrazide, 1-hydroxybenzotriazole, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, t...
Embodiment 2
[0042] An injectable cartilage repair hydrogel, comprising the following components in parts by weight: 45 parts of aminated chondroitin sulfate, 50 parts of oxidized dextran, and 5 parts of sodium alginate microspheres encapsulating growth factors. The hydrogel is in the form of a liquid gel.
[0043] The preparation method of the hydrogel in this example is the same as that in Example 1.
Embodiment 3
[0045] An injectable hydrogel for cartilage repair, comprising the following components in parts by weight: 49.5 parts of aminated chondroitin sulfate, 49.5 parts of oxidized dextran, and 1 part of sodium alginate microspheres encapsulating growth factors. The hydrogel is in the form of a liquid gel.
[0046] The preparation method of the hydrogel in this example is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com