Amorphous-state alloy catalyst and preparation method thereof
An amorphous alloy and catalyst technology, which is applied in the field of amorphous alloy catalyst and its preparation, can solve the problems of low hydrogen adsorption capacity, low hydrogenation selectivity, low final yield and the like, so as to avoid high cost, The effect of improving catalytic activity and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
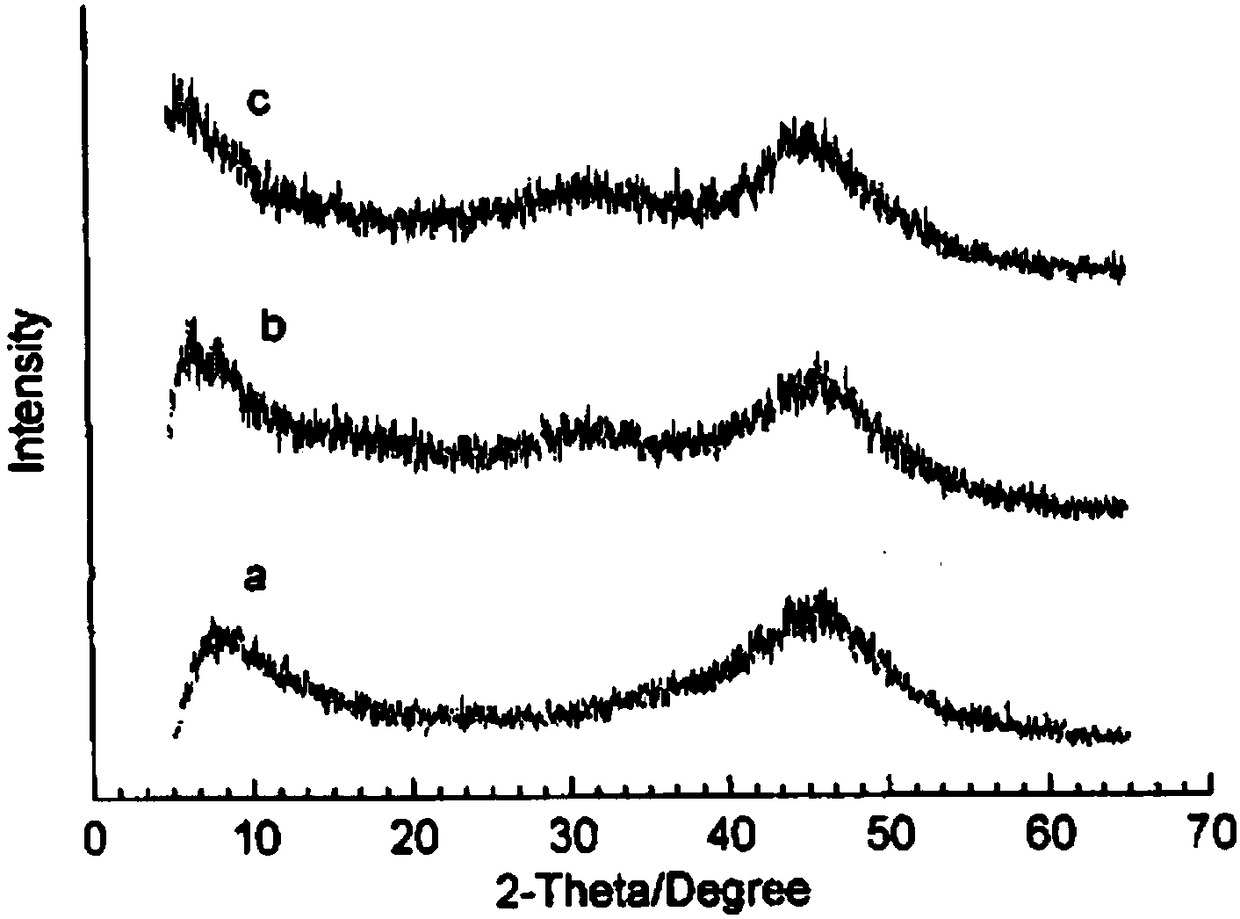
[0033] The invention provides an amorphous alloy catalyst and a preparation method thereof in one embodiment. Specifically, the preparation method of the amorphous alloy catalyst comprises the following steps:
[0034] Step S1: Dissolving the cobalt salt and the salt compound of M in water according to the ratio of the amount of cobalt and M being 100: (0 to 2) to obtain a mixed solution; wherein M is Fe, Cu, La, Ce, Ni, At least one of Zn and Mo.
[0035] Step S2: react the mixed solution with a reducing agent to obtain an amorphous alloy catalyst; wherein the reducing agent is KBH 4 And the aqueous solution of soluble hydroxide salt, the adding amount of reducing agent is according to the cobalt atom in the mixed solution and KBH 4 The ratio of the amount of the substance is 1:(3~4).
[0036] KBH 4 Under the conditions of the reaction between the solution and the metal salt with a certain reduction potential, a large amount of very fine H is rapidly released. 2 The bubb...
Embodiment 1
[0055] (1) Weigh a certain amount of CoCl 2 ·6H 2 O is dissolved in deionized water to make CoCl with a concentration of 1mol / L 2 Solution 1L.
[0056] (2) KBH 4 Dissolve in water with NaOH to form KBH containing 2mol / L 4 And 0.02mol / L NaOH reducing agent.
[0057] (3) CoCl prepared in step (1) under stirring 2 The reducing agent prepared in step (2) is slowly added dropwise into the solution, and black uniform particle precipitation is obtained in the reaction.
[0058] (4) After the reaction is completed, the precipitate is repeatedly washed with deionized water, and then washed with ethanol for 3 to 4 times to obtain a Co-B amorphous alloy catalyst, which is stored in absolute ethanol.
[0059] The Co-B amorphous alloy catalyst includes Co: 100 parts and B: 400 parts by substance amount.
Embodiment 2
[0061] (1) Weigh a certain amount of CoCl 2 ·6H 2 O and ZnCl 2 Dissolve in deionized water to make CoCl with a concentration of 1mol / L 2 solution and 0.01mol / L ZnCl 2 1L of the mixed solution.
[0062] (2) KBH 4 Dissolve in water with NaOH to form KBH containing 2mol / L 4 And 0.02mol / L NaOH reducing agent.
[0063] (3) CoCl prepared in step (1) under stirring 2 and ZnCl 2 Slowly add the reducing agent prepared in step (2) dropwise in the mixed solution, and react to obtain black uniform particle precipitation.
[0064] (4) After the reaction is completed, the precipitate is repeatedly washed with deionized water, and then washed with ethanol for 3 to 4 times to obtain a Co-B amorphous alloy catalyst, which is stored in absolute ethanol.
[0065] The Co-Zn-B amorphous alloy catalyst includes Co: 100 parts, Zn: 1 part and B: 400 parts according to the amount of substances; the material of the Zn element in the prepared Co-Zn-B amorphous alloy catalyst The percentage of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com