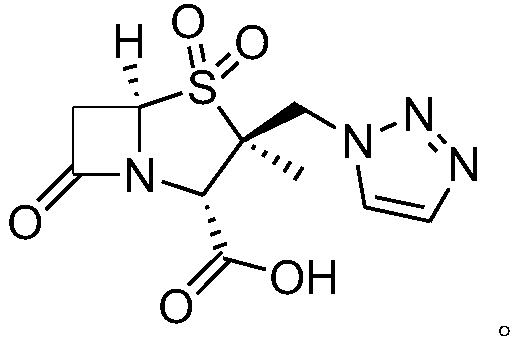
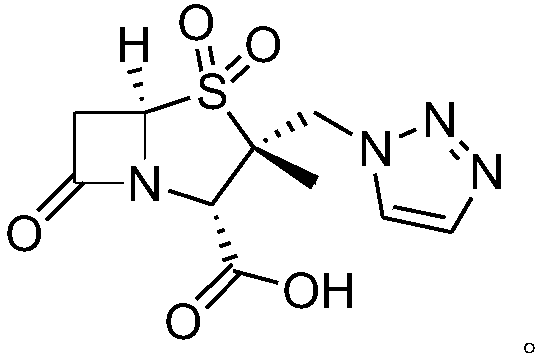
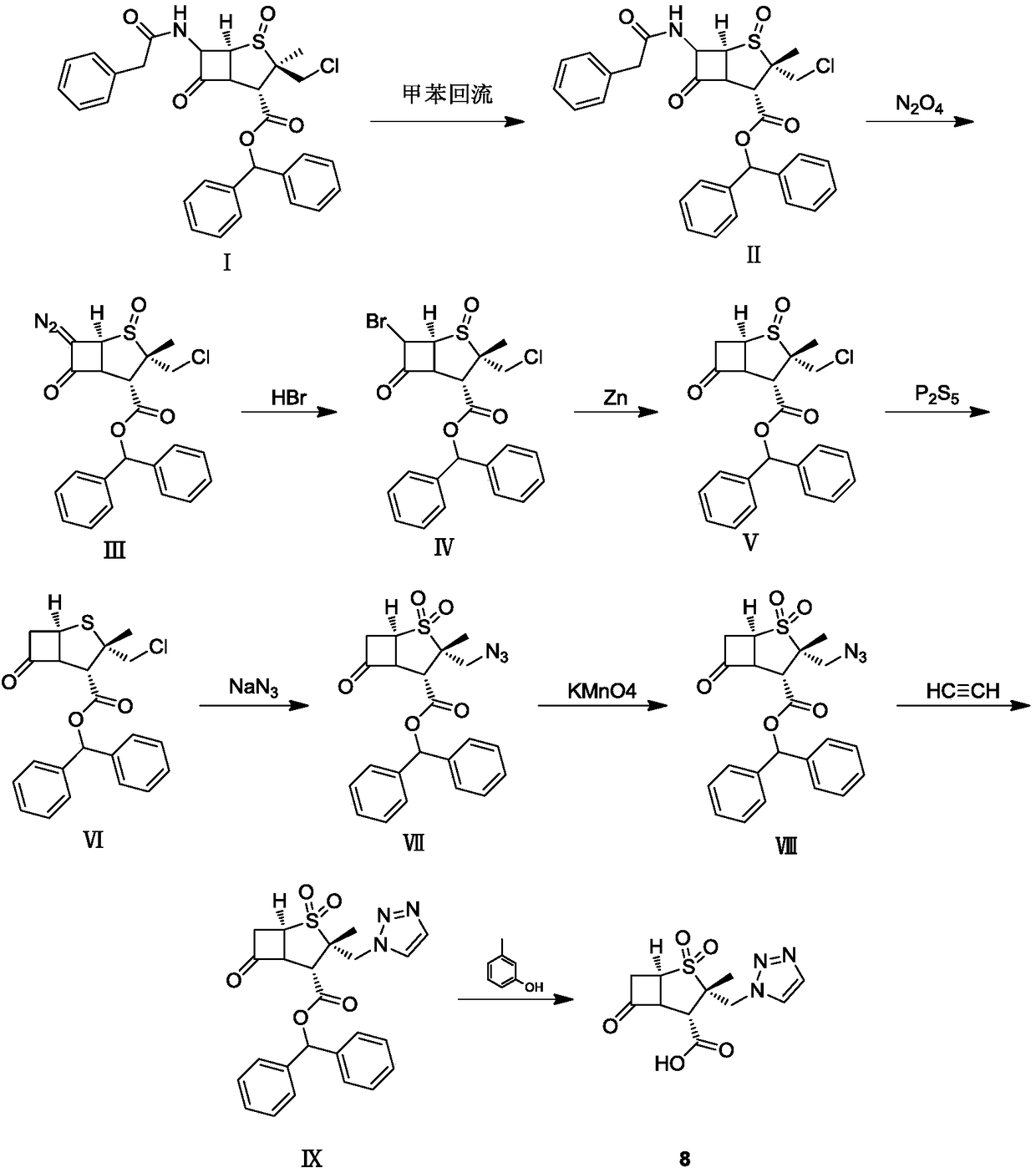
Synthetic method for tazobactam chiral isomer
A technology of chiral isomers and tazobactam, which is applied in the field of synthesis of tazobactam chiral isomers, can solve problems such as surplus raw materials and no product formation, and achieve low raw material costs and convenient post-processing , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
[0035] Add 100g of 6-APA to 366g of 50% dilute sulfuric acid, and cool to -5°C. Control the temperature at -10 to 0°C and add dropwise 123g sodium nitrite solution (43g sodium nitrite dissolved in 80g water). After the addition, the temperature was kept at -5-5°C for 1 hour. Control the temperature at -5°C to 5°C, add 177g sodium hypophosphite solution (57g sodium hypophosphite dissolved in 120g water) dropwise, and heat up to 20-30°C for 2 hours. After adding 1.2L of dichloromethane and stirring for 0.5 hour, stand still and separate into layers. The organic phase was washed with 80g saturated sodium bicarbonate solution and distilled to dryness under reduced pressure. Then methanol was added, stirred at 20-30°C for 3 hours, and filtered to obtain 82g of white solid (2S,5R)-3,3-dimethyl-7- Oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (compound 1), yield...
Embodiment 2
[0036] Example 2: (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid-4 oxide preparation
[0037] Add 45g (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and 800g two Chloromethane, cooling to -10~-5℃. Add sodium tungstate solution (0.03g sodium tungstate dissolved in 750g water), and then add 19g 50% hydrogen peroxide dropwise at -12~5°C. After dripping, keep the temperature at -10~5℃ for 2 hours. Filter, wash the filter cake with 42g of water, and dry under reduced pressure to obtain 44g of white solid (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0] Heptane-2-carboxylic acid-4 oxide (compound 2), yield 91%.
Embodiment 3
[0038] Example 3: (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid methyl ester-4 oxidation Preparation
[0039] Add 60g (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid-4 to the reaction flask Add 52 g of methyl iodide and 500 g of DMF, and stir at 20-30°C for 2 hours. The reaction solution was slowly poured into 1.5Kg of water, filtered, the filter cake was washed with 60g of water, and dried under reduced pressure to obtain 63g of off-white solid (2S,5R)-3,3-dimethyl-7-oxo-4-thia- 1-Azabicyclo[3.2.0]heptane-2-carboxylic acid methyl ester-4 oxide (Compound 3), the yield was 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com