Anti-human SAA (Serum Amyloid A) monoclonal antibody and preparation method and application thereof
A monoclonal antibody, antigen epitope technology, applied in biochemical equipment and methods, chemical instruments and methods, microorganism-based methods, etc., can solve problems such as no other reports, and achieve the effect of high affinity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
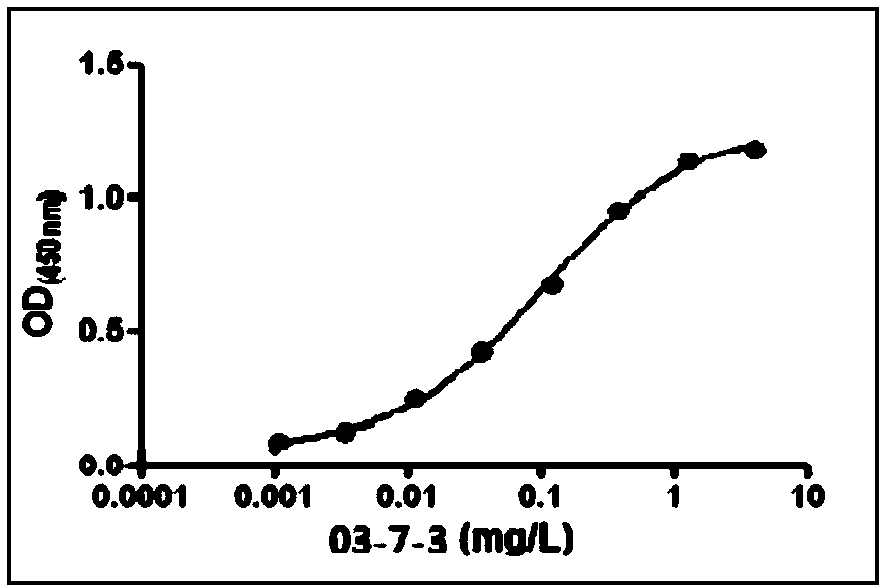
[0033] Example 1: Preparation and purification of SAA monoclonal antibody 03-7-3
[0034] 1. Material: His-SAA fusion protein, 8-week-old female BALB / c mice
[0035] 2. Methods and results
[0036] 2.1 Antigen preparation
[0037] 2.1.1 Construction of human SAA recombinant protein expression plasmid
[0038] The gene sequence of SAA (Gene ID: 6288) was searched in GenBank, and polymerase chain reaction (PCR) primers were designed according to the sequence. Human cDNA was used as a template to amplify the DNA fragment of the human SAA gene. The PCR product was detected by 1.0% agarose gel electrophoresis and the corresponding fragment was recovered with a gel recovery kit. The PCR amplified SAA gene fragment product was ligated into the expression vector pET28a( The His tag contained in the vector is used for purification), transformed into the TaKaRaDH5α host strain, and a single clone is picked for plasmid extraction and sequencing verification. The sequencing result is consistent w...
Embodiment 2
[0055] Example 2: Determination of the amino acid sequence of the variable region of SAA monoclonal antibody 03-7-3
[0056] 1. Material: Trizol (Invitrogen), the primers are synthesized by Shenggong Bioengineering Company, and the reverse transcription and PCR reagents are purchased from
[0057] TaKaRa company.
[0058] 2. Methods and results:
[0059] 2.1 Total RNA extraction and first strand synthesis of cDNA
[0060] The hybridoma cells in the logarithmic growth phase were collected, and total RNA was extracted according to the operating procedure of Trizol. Use spectrophotometer and agarose gel electrophoresis to qualitatively and quantitatively identify total RNA.
[0061] According to PrimeScript of TaKaRa TM II Description of 1st Strand cDNA Synthesis Kit Synthesize cDNA.
[0062] 2.2 SAA monoclonal antibody 03-7-3 heavy chain variable region (VH) and light chain variable region (VL) gene fragment amplification and sequencing
[0063] According to TaKaRa company Taq enzyme inst...
Embodiment 3
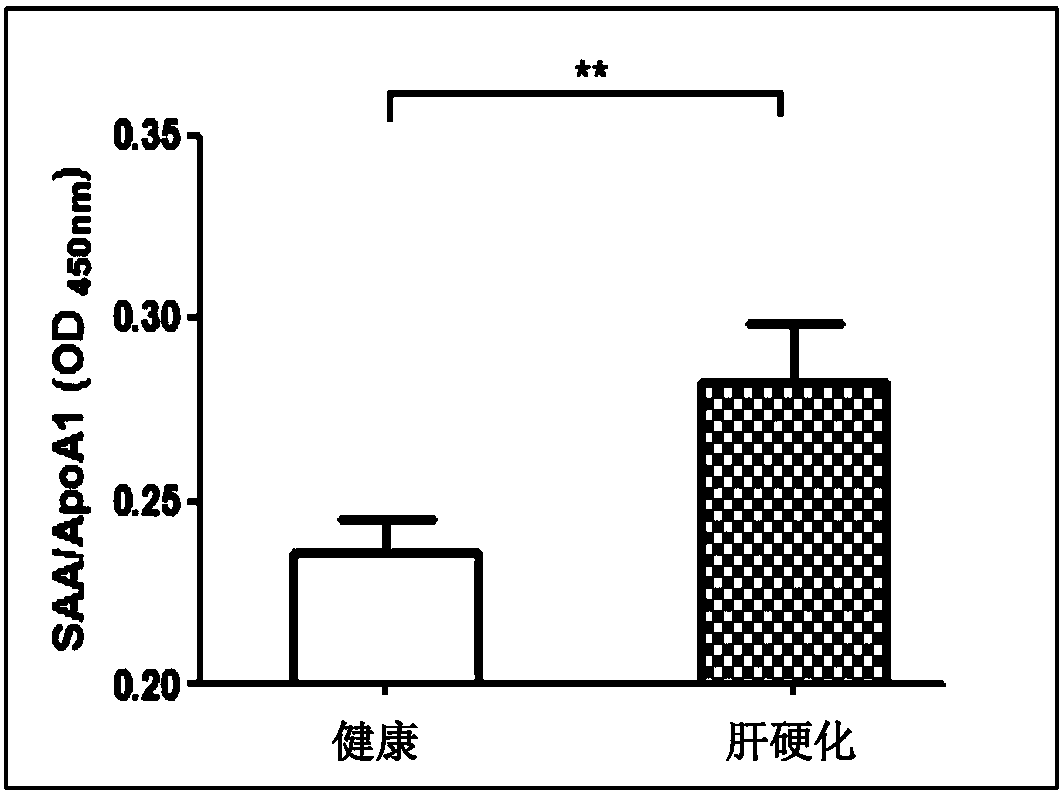
[0067] Example 3: SAA-ApoA1-HDL complex detection method established based on SAA monoclonal antibody 03-7-3 and its clinical application evaluation in the risk and diagnostic value of liver cirrhosis
[0068] 1. Material:
[0069] SAA monoclonal antibody 03-7-3; serum samples from healthy people on physical examination (normal liver function and two pairs and a half negative for hepatitis B) and patients with liver cirrhosis.
[0070] 2. Methods and results:
[0071] 2.1 Collection of clinical samples and related clinical information:
[0072] Collect 12 serum samples of healthy control group and 12 serum samples of liver cirrhosis group. The healthy control group is a healthy population with physical examination. The liver function indexes (alanine aminotransferase, aspartate aminotransferase, total bilirubin and direct indirect bilirubin, etc.) are all within the normal physiological value range, and hepatitis B is two and a half pairs It was negative, and there was no history of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com