Reagent kit and screening method for screening carbapenem resistant enterobacteria from excrement and urine sample
A technology for screening carbapenems and enterobacteriaceae, applied in the fields of molecular biology and medical microbiology, to achieve convenient and quick use, increase positive rate, and reduce fungal interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
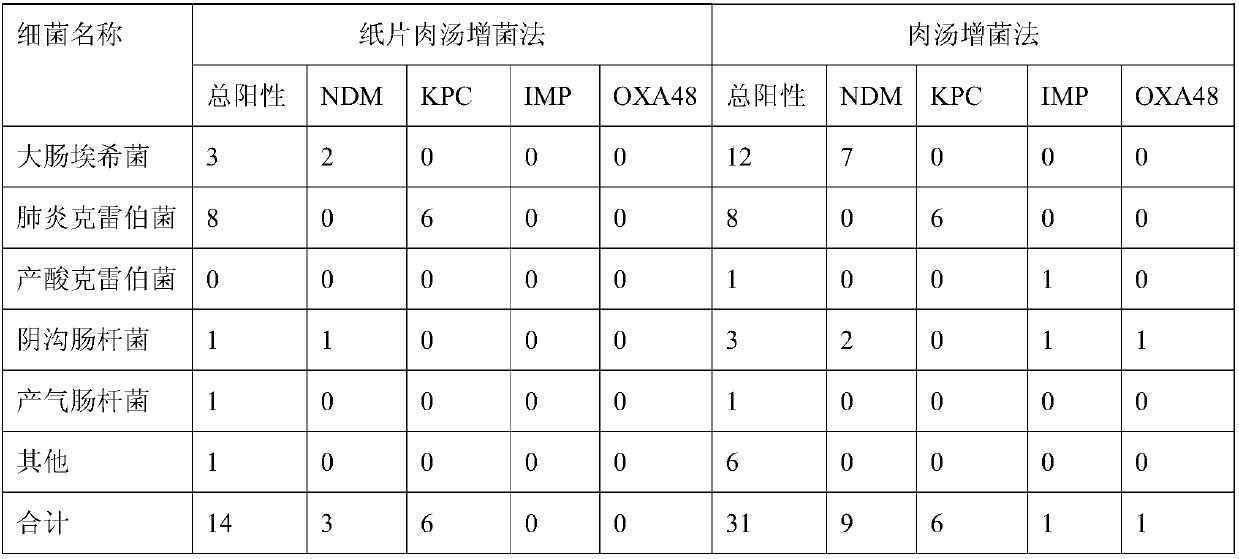
[0020] Embodiment 1 (broth enrichment method)
[0021] 1 Sample inoculation and enrichment for the first time: pick an appropriate amount of feces (about the size of a matchhead) with a cotton swab and inoculate into 5ml of feces enrichment broth, and incubate in a 35°C incubator for 8-12 hours.
[0022] 2. The second drug-containing plate screening of the sample: pick a loop of the enrichment solution after the first broth enrichment and inoculate it on a Chinese blue plate with 0.3ug / ml meropenem, and incubate in a 35°C incubator for 8- 12h.
[0023] 3. From the colonies grown on the plate containing the drug, determine whether there is CRE by the following methods:
[0024] 3.1 Bacterial identification: Pick a single colony from the drug-containing plate and spread it evenly on the target plate for mass spectrometry. After drying, add 1 μl of matrix solution to cover the target point and let it dry naturally. Put the target plate into the mass spectrometer to start the id...
Embodiment 2
[0027] Embodiment 2 (CDC method)
[0028] 1 Sample inoculation: Pick up an appropriate amount of feces (about the size of a matchhead) with a cotton swab and inoculate into 5ml of fecal enrichment broth, add a piece of ertapenem disc (10ug / ml), and incubate in a 35°C incubator for 8-12h.
[0029] 2. Transfer of the enrichment solution: Pick a loop of the enrichment solution after the first enrichment of the broth containing the drug and inoculate it on a Chinese blue plate commonly used in clinical practice (the Chinese blue plate in this example does not contain meropenem), 35 Incubate in an incubator for 8-12 hours.
[0030] 3. From the colonies grown on the plate containing the drug, determine whether there is CRE by the following methods:
[0031] 3.1 Bacterial identification: Pick a single colony from the drug-containing plate and spread it evenly on the target plate for mass spectrometry. After drying, add 1 μl of matrix solution to cover the target point and let it dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com