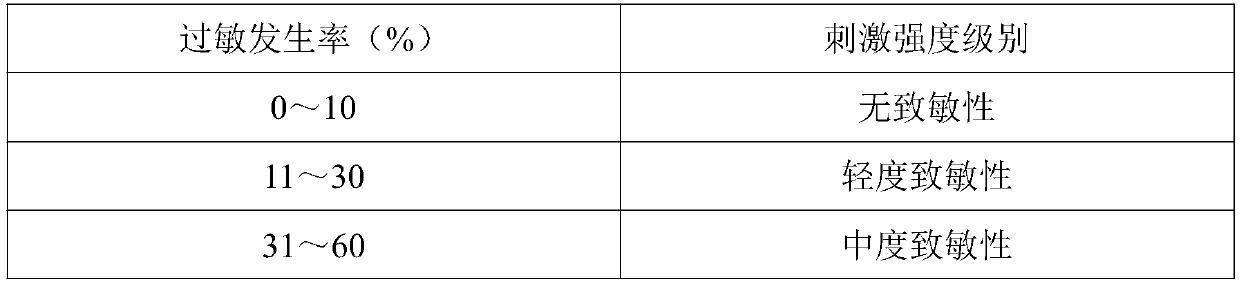
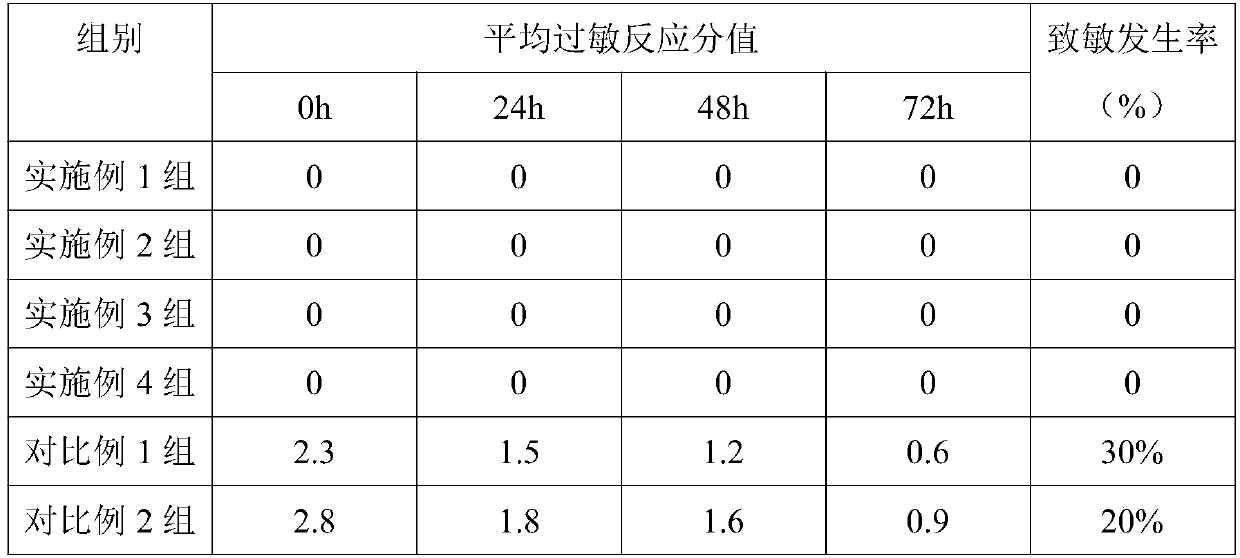
Frozen analgesic aerosol and preparation method thereof
An aerosol and analgesic technology, which is applied in the field of frozen analgesic aerosol and its preparation, can solve the problems of high cost, complicated preparation method, and large human body irritation, and achieve lower phase transition temperature and strong skin penetration Action, Diffusion resistance reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 A kind of frozen analgesic aerosol
[0031] The frozen analgesic aerosol is composed of the following components and their weight percentages: pharmaceutical grade dimethyl ether 40.0%, 1-fluoro-3,3,3-difluoropropene 56%, skin penetration enhancer 0.5%, Analgesics 2.0% and Hyaluronic Acid 1.5%.
[0032] The skin penetration enhancer is composed of azone, menthol and chondroitin sulfate in a weight ratio of 4:9:3.
[0033] The analgesic is composed of fumaric tetrahydropalmatine, the extract of Limonium chinensis and glucosamine in a weight ratio of 13:5:12.
[0034] The preparation method of described frozen analgesic aerosol comprises the following steps:
[0035] S1: Add trans-1-fluoro-3,3,3-difluoropropene into trans-1-fluoro-3,3,3-difluoropropene with transdermal enhancer, analgesic and hyaluronic acid, and stir at high speed until the mixture becomes a microemulsion with a particle size of 1 μm solution;
[0036] S2: Pour the micron-level microemul...
Embodiment 2
[0037] Embodiment 2 A kind of frozen analgesic aerosol
[0038]The frozen analgesic aerosol is composed of the following components and their weight percentages: 1,3,3,3-tetrafluoropropene 55.0%, trans-1-fluoro-3,3,3-difluoropropene 39.0%, penetration enhancer 1.2%, analgesic 3.0% and skin tissue repairing agent 1.8%.
[0039] The skin penetration enhancer is composed of azone, menthol and chondroitin sulfate in a weight ratio of 9:7:2.
[0040] The analgesic is composed of fumaric tetrahydropalmatine, the extract of Limonium chinensis and glucosamine in a weight ratio of 16:3:7.
[0041] The skin tissue repairing agent is composed of Eucommia ulmoides extract, allantoin and aloin in a weight ratio of 3:1:1.
[0042] The preparation method of described frozen analgesic aerosol comprises the following steps:
[0043] S1 Add trans-1-fluoro-3,3,3-difluoropropene into trans-1-fluoro-3,3,3-difluoropropene, add transdermal enhancer, analgesic and skin tissue repairing agent, and ...
Embodiment 3
[0045] Embodiment 3 A kind of frozen analgesic aerosol
[0046] The frozen analgesic aerosol is composed of the following components and their weight percentages: 70.0% of liquefied petroleum gas, 22.2% of trans-1-fluoro-3,3,3-difluoropropene, and 1.8% of transdermal accelerator %, analgesics 3.5% and aloin 2.5%.
[0047] The skin penetration enhancer is composed of azone, menthol and chondroitin sulfate in a weight ratio of 13:5:1.
[0048] The analgesic is composed of fumaric tetrahydropalmatine, the extract of Limonium chinensis and glucosamine in a weight ratio of 18:1:4.
[0049] The preparation method of described frozen analgesic aerosol comprises the following steps:
[0050] S1 Add trans-1-fluoro-3,3,3-difluoropropene into trans-1-fluoro-3,3,3-difluoropropene, add transdermal enhancer, analgesic and aloe vera, and stir at high speed until the mixture becomes a microemulsion solution with a particle size of 10 μm ;
[0051] S2: Pour the micron-level microemulsion s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com