Ammonia regeneration and recycling process for ammonia desulfurization
An ammonia-based desulfurization and process technology, applied in the field of ammonia-based desulfurization, can solve problems such as unorganized emission of environmental pollution, poisoning of operators around the regeneration pool, etc., and achieve the effect of avoiding major poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
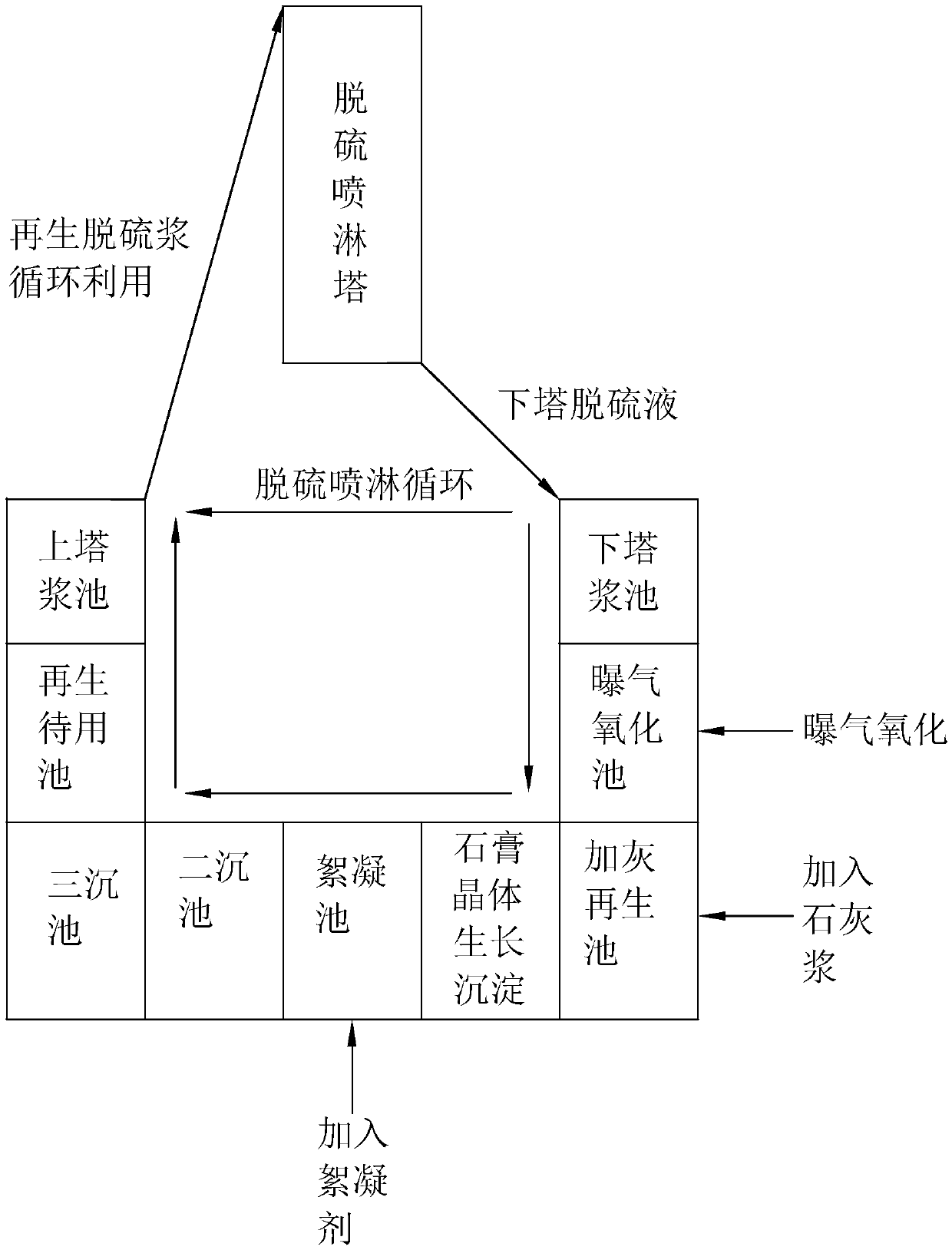
[0057] The ammonia regeneration and recycling process of ammonia desulfurization includes the following process steps:
[0058] (1) Part of the desulfurization liquid containing ammonium sulfite and ammonium bisulfite undergoes redox reaction after aeration:
[0059] 2(NH 4 ) 2 SO 3 +O 2 →2(NH 4 ) 2 SO 4 , 2NH 4 HSO 3 +O 2 →2NH 4 HSO 4 ;
[0060] (2) The oxidized desulfurization liquid and milk of lime undergo a displacement reaction under stirring:
[0061] (NH 4 ) 2 SO 4 +Ca(OH) 2 →2NH 3 + CaSO 4 2H 2 O.
[0062] 2NH 4 HSO 4 +Ca(OH) 2 →(NH 4 ) 2 SO 4 +CaSO 4 2H 2 O;
[0063] (3) Calcium sulfate dihydrate is separated to obtain regenerated ammonia solution after flocculation and precipitation;
[0064] (4) The regenerated ammonia solution is mixed with another part of the desulfurization liquid containing ammonium sulfite and ammonium bisulfite:
[0065] NH 3 + NH 4 HSO 3 →(NH 4 ) 2 SO 3 ;
[0066] (5) The desulfurization liquid is mixed ...
Embodiment 2
[0074] The difference between embodiment 2 and embodiment 1 is that the control conditions of each process step are different. Specifically, in step (1), the oxidation rate of ammonium sulfite and ammonium bisulfite is not lower than 98%, and the aeration oxygen-sulfur molar ratio is not lower than 1.5. In step (2), the pH value of the replacement reaction system is controlled to be kept at 6.8. In steps (4) and (5), the regenerated desulfurization liquid 6.2 is controlled by controlling the amount of regenerated ammonia water and additional ammonium bicarbonate aqueous solution. In step (6), the pH value of the desulfurization slurry after the desulfurization reaction is maintained at 6.2 by controlling the amount of regenerated ammonia water and supplementary ammonium bicarbonate aqueous solution. In step (2), the weight percent concentration of milk of lime is 10%. In step (2), the stirring speed of the reaction system is not lower than 70 rpm. In step (3), polyacrylamid...
Embodiment 3
[0076] The difference between embodiment 3 and embodiment 1 is that the control conditions of each process step are different. Specifically, in step (1), the oxidation rate of ammonium sulfite and ammonium bisulfite is not lower than 98%, and the aeration oxygen-sulfur molar ratio is not lower than 1.5. In step (2), the pH value of the replacement reaction system is controlled to remain at 6.9. In steps (4) and (5), the regenerated desulfurization liquid 5.8 is controlled by controlling the amount of regenerated ammonia water and supplemented ammonium bicarbonate aqueous solution. In step (6), the pH value of the desulfurized slurry after the desulfurization reaction is maintained at 4.5 by controlling the amount of regenerated ammonia water and supplementary ammonium bicarbonate aqueous solution. In step (2), the weight percent concentration of milk of lime is 30%. In step (2), the stirring speed of the reaction system is not lower than 70 rpm. In step (3), polyacrylamide ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com