Synthesis of Spiro Bisboron Catalyst and Its Application in Hydrogenation
A catalyst and spiro ring technology, which is applied in the synthesis of spiro diene compounds, can solve the problems of limited chiral boron catalysts, achieve the effects of solving heavy metal residues, mild reaction conditions, and a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
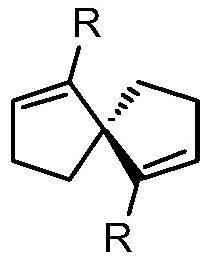
[0024] Embodiment 1: the synthesis of chiral spirodiene compound 3a
[0025]
[0026] The first step: resolution of spiro[4.4]-1,6-nonanedione
[0027] (R)-Phenylethylaminooxalazide (4.15g, 2eq) was added to a 250mL dry round bottom flask, followed by 1.52g of racemic spiro[4.4]-1,6-nonanedione and a small pellet Add 120mL of anhydrous dichloromethane to iodine element under the protection of argon, install a water separator and a reflux condenser, and heat to reflux overnight. After the reaction, the system was cooled to room temperature, and the insoluble solid was removed by diatomaceous earth filtration, and the dichloromethane was removed by rotary evaporation under reduced pressure to obtain a yellow solid crude product, which was recrystallized and purified from anhydrous ethanol to obtain 3.5 g of a white powdery solid dihydrazone compound intermediate , yield 66%.
[0028] 2.0 g of the dihydrazone compound intermediate was added into a 500 mL eggplant-shaped bott...
Embodiment 2
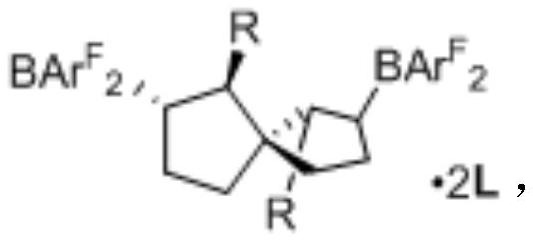
[0034] Example 2: Synthesis and identification of spirocyclic bis-boron catalyst 4a
[0035]
[0036] In the glove box, 3a (13.6mg, 0.05mmol), HB(C 6 f 5 ) 2 (34.6mg, 0.1mmol) and toluene (1.0mL), react at 25°C for 15 minutes. Isoquinoline (25.8mg, 0.2mmol) and toluene (0.5mL) were added again, and the reaction was continued at 25°C for 30 minutes. After the reaction, the toluene was distilled off under reduced pressure to obtain a white powdery solid, and the internal standard CH was added. 2 Br 2 ,pass 1 H NMR judged that the NMR yield was 95%. The crude product was recrystallized from dichloromethane and n-hexane to obtain product 4a·2L. 1 H NMR (400MHz, CD 2 Cl 2 )δ8.74(s, 2H), 8.04(d, J=6.8Hz, 2H), 7.83(t, J=7.5Hz, 2H), 7.74(d, J=8.1Hz, 2H), 7.62(t, J=7.4Hz, 2H), 7.59-7.30(m, 8H), 7.16(t, J=7.1Hz, 2H), 6.87(br, 2H), 6.69(br, 2H), 2.92(t, J=9.6 Hz, 2H), 2.32-2.18(m, 2H), 2.14(d, J=9.0Hz, 2H), 1.80-1.54(m, 4H), 0.86(dd, J=19.2, 11.5Hz, 2H); 13 C NMR (101MHz, ...
Embodiment 3
[0037] Embodiment 3: Synthesis of (R)-2-methyl-1,2,3,4-tetrahydroquinoline (P1)
[0038]
[0039] In a glove box filled with nitrogen, the chiral spirodiene compound 3a (3.4mg, 0.0125mmol, 5mol%) and HB(C 6 f 5 ) 2 (8.65mg, 0.025mmol, 10mol%) was added to a 10mL small test tube, 2mL of trifluorotoluene was added to dissolve, and the reaction was carried out at 25°C for 15 minutes. The system was cooled to room temperature, and then 2-methylquinoline S1 (35.8mg, 0.25mmol) and 3mL of benzotrifluoride were added, and then the test tube was transferred to the autoclave, and the hydrogen was replaced three times, and finally filled with hydrogen to 50bar,- Reaction at 20°C for 24h. After the reaction, hydrogen was released, the solvent was removed by rotary evaporation, and the residue was separated and purified by silica gel column chromatography to obtain the hydrogenated product P1, a colorless oily liquid, with a yield of 97% and an enantioselectivity of 90% ee. 1 H NMR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com