Acetohydroxyacid synthase mutant for improving L-valine synthesis efficiency
A technology of acetohydroxyacid synthase and mutants, which is applied in the field of metabolic engineering, can solve the problems of poor selectivity of the second substrate, etc., and achieve the effect of improving specificity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Construction of acetohydroxyacid synthase expression strain
[0025] Primers were designed according to the acetohydroxyacid synthase gene sequence of Corynebacterium glutamicum in NCBI, as follows
[0026] Upstream primer: CGC GGATCC AGAAGGAGGTATAGTAGTGAATGTGGCAGCTTCTCAACA
[0027] Downstream primer: G GAATTC TTAGATCTTGGCCGGAG
[0028] Link the PCR product of acetohydroxyacid synthase to the plasmid pJYW-4 (the construction method is disclosed in Construction of a novel expression system for use in Corynebacterium glutamicum), transform E.coliJM109 competent cells, and culture in 1 mL of LB medium For 40 minutes, take 50 μl of the bacterial solution and spread it on a solid LB plate containing kanamycin, incubate at 37°C for 10 hours, pick the positive colony, and culture it overnight on a shaker at 37°C, extract the plasmid, and verify that the enzyme digestion is correct. Lin company sequenced to obtain the construction of recombinant expression p...
Embodiment 2
[0030] Example 2: Obtaining of Mutants with Enhanced Substrate Specificity
[0031] Using site-directed mutagenesis, primers were designed as follows:
[0032] F: ATGCTTTCCAGGAAGTCGATATCCGCGGC
[0033] R: GCCGCGGATATCGACTTCCTGGAAAGCAT
[0034] Using the constructed plasmid pJYW4-ilvBN as a template, PCR was carried out to mutate the alanine at position 138 of acetohydroxyacid synthase into valine. The PCR conditions were 95°C for 5min, 34 cycles (95°C for 5min, 55°C for 30min, 68°C for 10min), 72°C for 10min, and 4°C for incubation. PCR amplification system: template 0.5 μl, upstream and downstream primers 0.5 μl, 5×PS Buffer 5 μl, dNTPmix 2 μl, primeSTAR 0.25 μl, ddH 2 O 16.25 μl. Take 5 μl for nucleic acid electrophoresis verification, add 1 μl DpnI to the rest to remove the template, and carry out transformation at 37°C for 1 hour. The transformant was sequenced by Tianlin Company, and the transformant was named pJYW4-ilvBN-A138V. By the electroporation method in Exam...
Embodiment 3
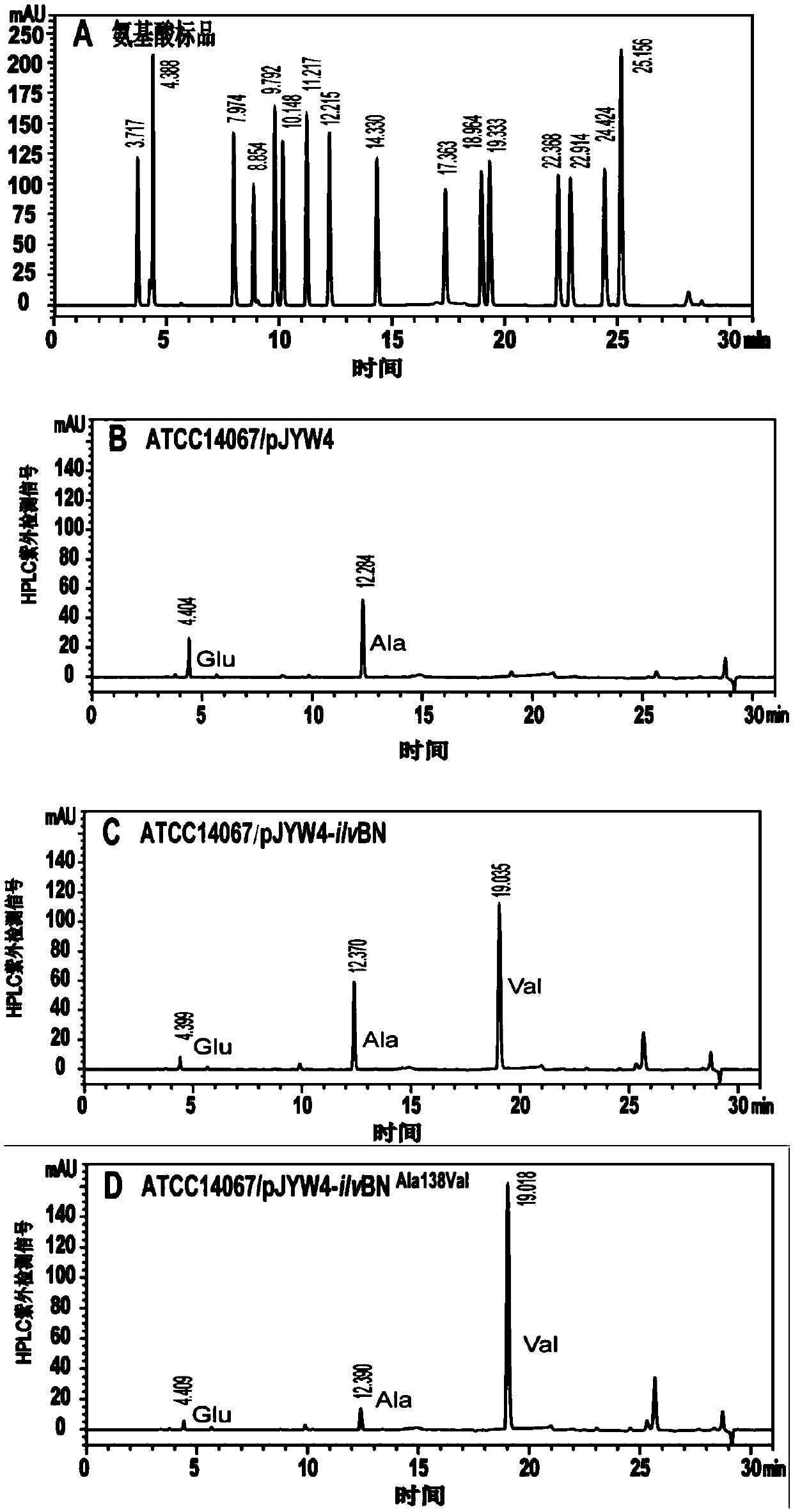
[0035] Example 3: High-specificity acetohydroxyacid synthase assay in glutamic acid rod-shaped rod enzyme activity
[0036] The strains sequenced correctly in Examples 1 and 2, and the starting strain ATCC 14067pJYW4 were respectively streaked on a solid LBB plate under the same conditions to activate the strains, cultured at 30°C for 48 hours, and the activated strains were divided into three mung bean-sized amounts, Transfer 50mL seed medium, culture at 30°C 200rpm for 12h, until the initial OD 562nm Transfer to 50mL fermentation medium at 1 o'clock, ferment at 30°C and 200rpm for 36h, take 3mL of fermentation broth to collect bacterial cells by centrifugation, and resuspend to OD with phosphate buffer 562nm If it is 7, the cells are sonicated for 20 minutes with an ultrasonic breaker, centrifuged at 12000 rpm for 15 minutes, and the supernatant is the acetohydroxyacid synthase enzyme solution. Using the enzyme activity detection method, when the pH is 5.8, the specific enzy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com