Method for detecting related substances in pharmaceutical preparation containing citicoline sodium
A technology of citicoline sodium and a detection method, applied in the field of drug detection, can solve problems such as effective detection and separation of substances that cannot be used, and achieve the effects of good applicability, good durability, high accuracy and precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Instrument and chromatographic conditions:
[0024] Japan Shimadzu LC-20A; octadecylsilane bonded silica gel column (Ultimate XB-C18 250×4.6mm, 5μm); column temperature is 5℃; mobile phase is phosphate buffer [0.1mol / L diphosphate Potassium hydrogen solution and tetrabutylammonium solution (take 0.01mol / L tetrabutylammonium hydroxide solution and adjust the pH value to 5.2 with phosphoric acid) and mix in equal amounts]-methanol (95:5); flow rate 1.0ml / min; detection wavelength: 276nm ; Injection volume 10 μl.
[0025] experiment procedure:
[0026] Accurately weigh 15 mg each of 5'-cytidylic acid, urophosphorylcholine, cytosine, cytidine, uridine, and 5'-uridine reference substance, put them in 100ml measuring bottles, add water to dissolve and Dilute to the mark, shake well, and prepare the stock solution of each impurity reference substance; accurately weigh 0.1 g of the citicoline sodium reference substance, put it in a 100ml measuring bottle, accurately add 2ml e...
Embodiment 2
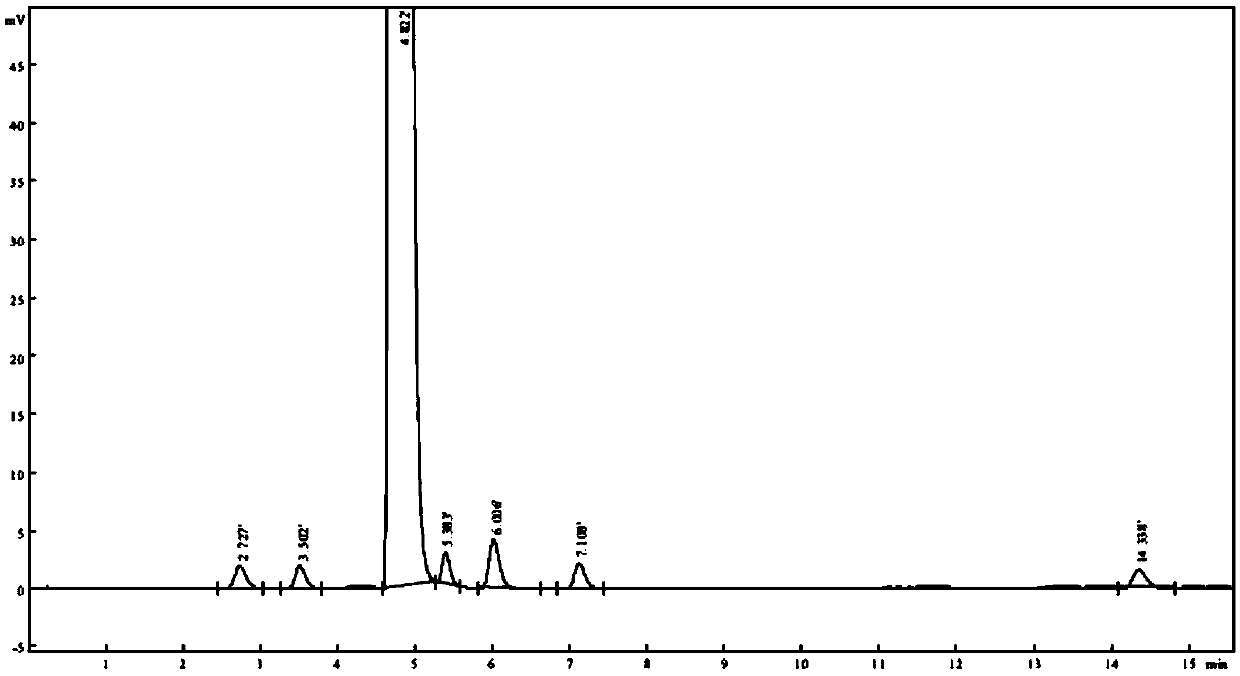
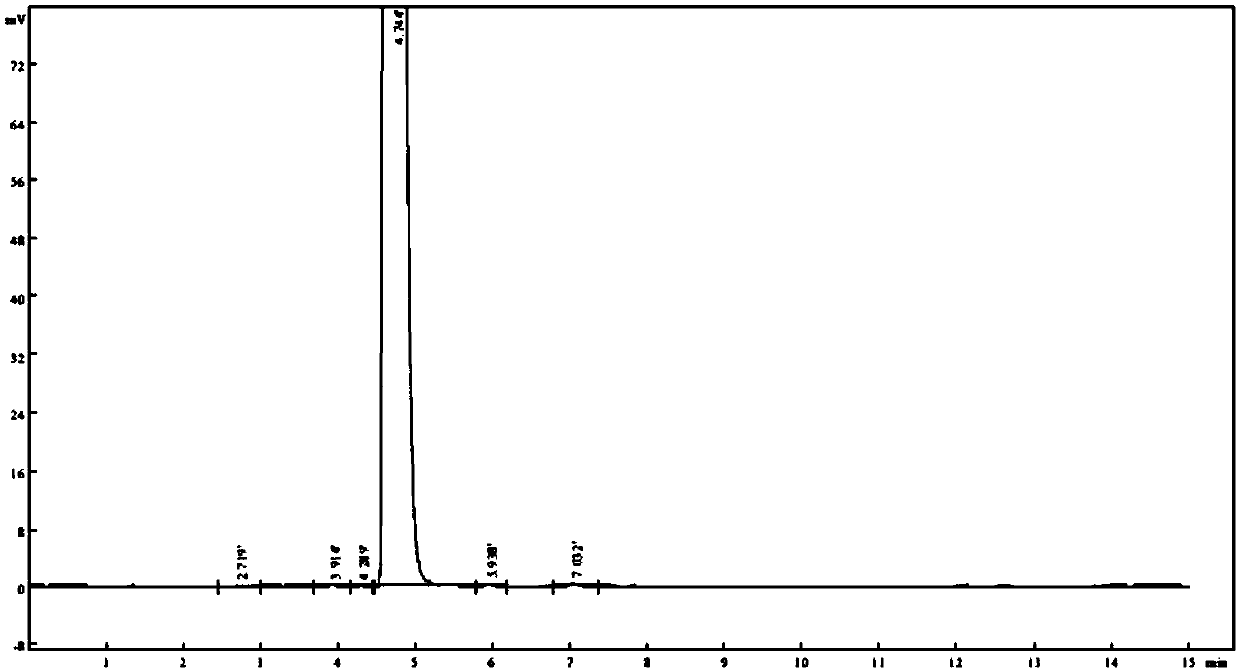
[0033] Change flow velocity (± 0.1ml / min), then according to the method for embodiment 1, need testing solution is detected, take the number of impurity in the chromatogram of record and the degree of separation of main component peak and adjacent impurity as index, investigate different The separation effect of flow rate in a chromatographic system. see results Figure 3 ~ Figure 4 and Table 1.
[0034] The detection result of the need testing solution of table 1 different flow rates
[0035] Flow rate (ml / min)
[0036] The above test results show that the number of detected impurities is consistent when the flow rate is in the range of 0.9-1.1ml / min, and the resolution of each impurity is above 1.5. In a preferred solution, the flow rate is 1.0ml / min.
Embodiment 3
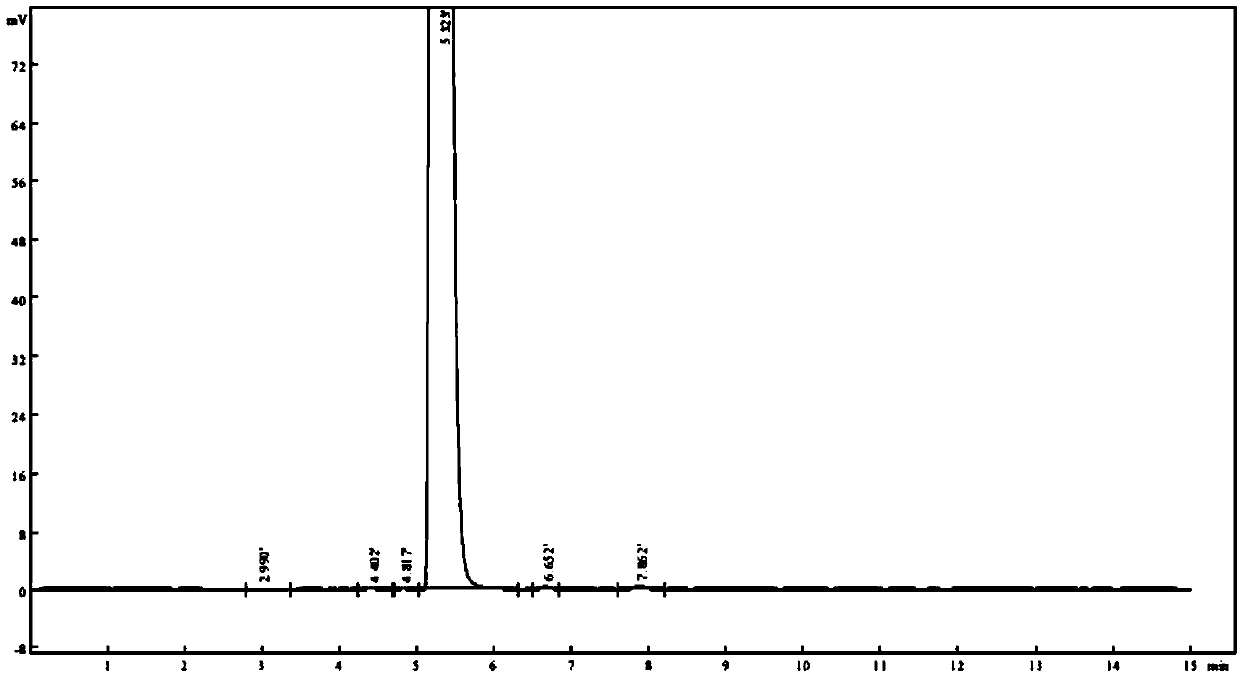
[0038] Change the pH value of the mobile phase phosphate buffer, prepare phosphate buffer with different pH values, and then detect the solution of the test product according to the method of Example 1, and record the number of impurities in the chromatogram and the peak and phase of the main component. The separation degree of adjacent impurities was used as an index to investigate the separation effect of phosphate buffer with different pH values in the chromatographic system. see results Figure 5 ~ Figure 6 and Table 2.
[0039] The detection result of the need testing solution of table 2 different pH value phosphate buffered saline
[0040] pH of Phosphate Buffer
[0041] The above test results show that when the pH of the mobile phase phosphate buffer is in the range of 5.0 to 5.2, the number of detected impurities is consistent, and the resolution of each impurity is above 1.5. In a preferred scheme, the pH of the mobile phase phosphate buffer is 5.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com