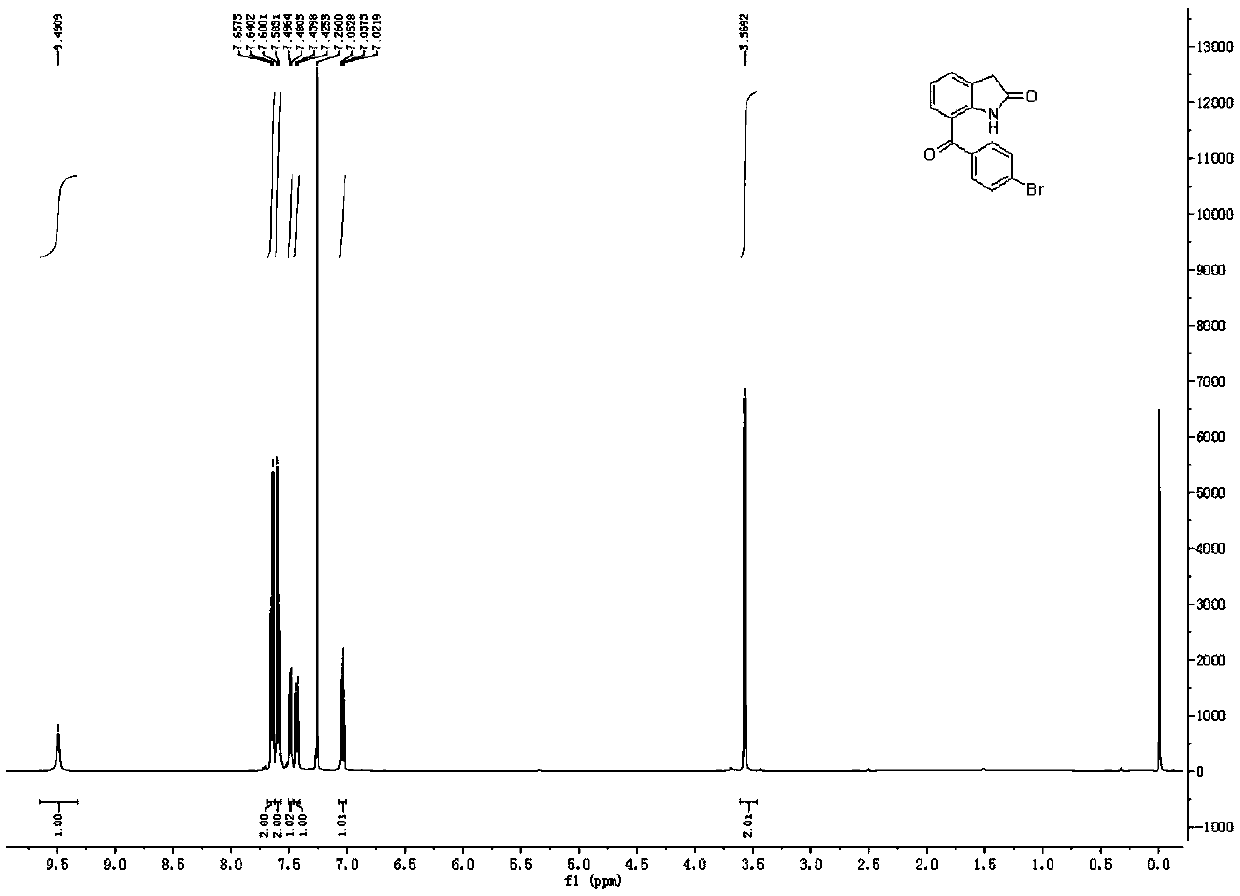
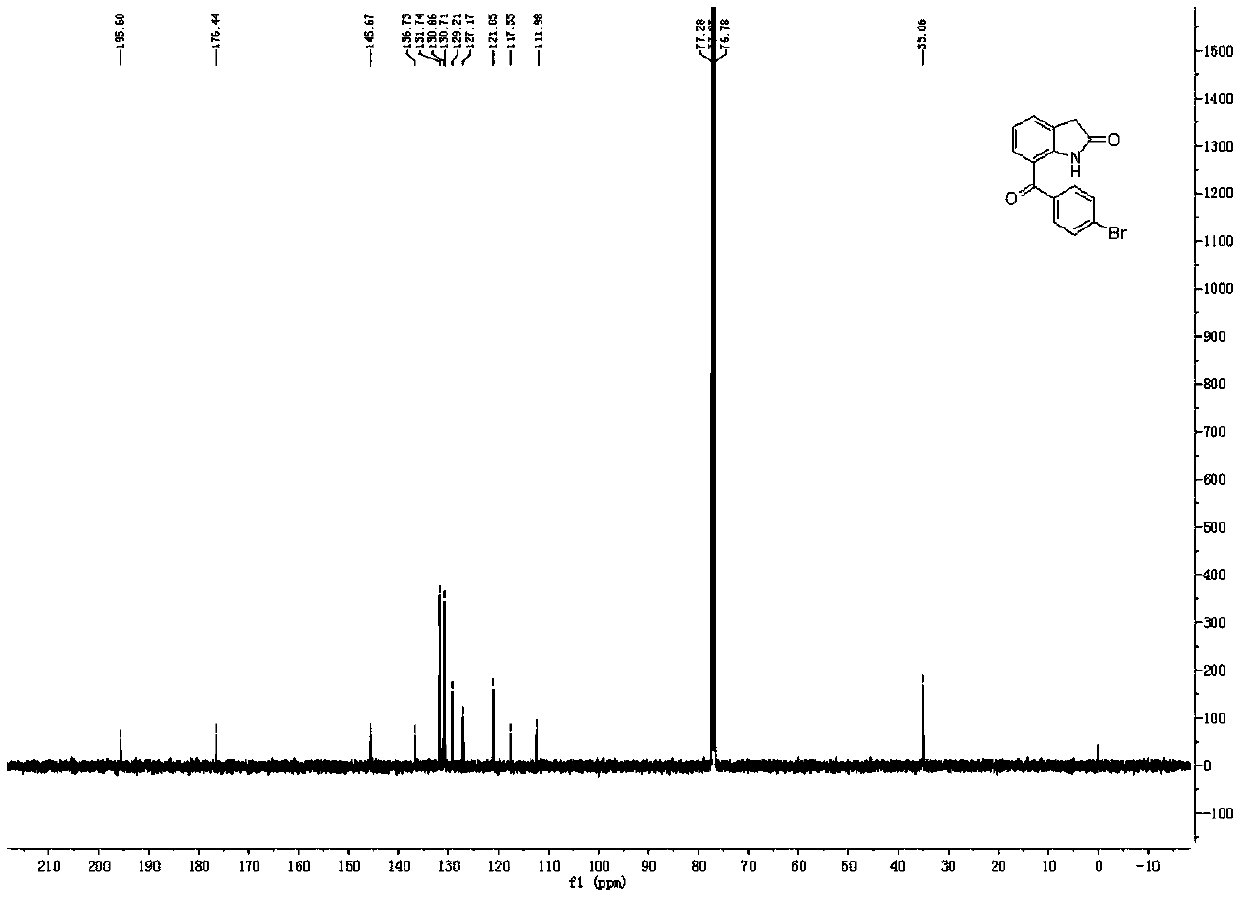
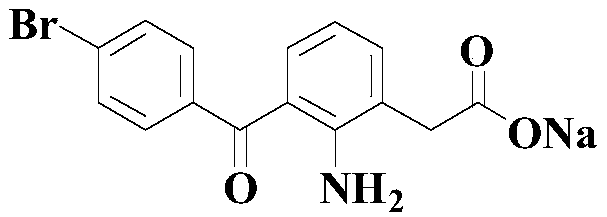
Synthetic method of BRONUCK intermediate 7-(p-bromobenzoyl)indole-2-one
A bromobenzoyl and synthetic method technology, applied in the field of organic chemical synthesis, can solve problems such as difficult to accept metal species addition, difficult to realize cyano addition reaction, increase of electron cloud density of benzene ring, etc., and achieve significant economic value and the effect of application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The reaction formula is as above, and the concrete reaction process is as follows:
[0053] At room temperature, add 100mmol 7-cyanoindol-2-one, 200mmol p-bromophenylboronic acid, 15mmol nickel catalyst Ni(dppe)Cl to an appropriate amount of solvent (2-methyltetrahydrofuran) in the reaction vessel 2 And 400mmol trifluoromethanesulfonic acid, then stirred and warmed up to 70 ° C, and stirred at this temperature for 24 hours.
[0054] After the reaction was completed, the reaction mixture was rotary evaporated to dryness, then a sufficient amount of ethyl acetate was added to dissolve it, and then an appropriate amount of saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride was added for washing, and the organic layer and the aqueous solution were separated. Aqueous layer, after the aqueous layer was extracted 3 times with ethyl acetate, the organic layer was combined (that is, the organic layer obtained by combining the organic layer washed with the...
Embodiment 2
[0056] The reaction formula is as above, and the concrete reaction process is as follows:
[0057] At room temperature, add 100mmol 7-cyanoindol-2-one, 400mmol p-bromophenylboronic acid, 5mmol nickel catalyst Ni(dppe)Cl to an appropriate amount of mixed solvent (2-methyltetrahydrofuran) in the reaction vessel 2 and 500 mmol trifluoromethanesulfonic acid, then stirred and heated to 100 ° C, and stirred at this temperature for 12 hours.
[0058] After the reaction was completed, the reaction mixture was rotary evaporated to dryness, then a sufficient amount of ethyl acetate was added to dissolve it, and then an appropriate amount of saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride was added for washing, and the organic layer and the aqueous solution were separated. Aqueous layer, after the aqueous layer was extracted 3 times with ethyl acetate, the organic layer was combined (that is, the organic layer obtained by combining the organic layer washed with...
Embodiment 3
[0060] The reaction formula is as above, and the concrete reaction process is as follows:
[0061] At room temperature, add 100mmol 7-cyanoindol-2-one, 300mmol p-bromophenylboronic acid, 10mmol nickel catalyst Ni(dppe)Cl to an appropriate amount of mixed solvent (2-methyltetrahydrofuran) in the reaction vessel 2 And 450mmol trifluoromethanesulfonic acid, then stirred and warmed up to 80 ° C, and stirred at this temperature for 18 hours.
[0062] After the reaction was completed, the reaction mixture was rotary evaporated to dryness, then a sufficient amount of ethyl acetate was added to dissolve it, and then an appropriate amount of saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride was added for washing, and the organic layer and the aqueous solution were separated. Aqueous layer, after the aqueous layer was extracted 3 times with ethyl acetate, the organic layer was combined (that is, the organic layer obtained by combining the organic layer washed wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com