2,5-thiophenedicarboxylic acid-based copolyester and preparation method thereof
A technology of thiophenedicarboxylic acid-based copolyester and thiophenedicarboxylic acid diester, which is applied in the field of polyester materials, can solve the problems of high glass transition temperature, poor material toughness, large production demand, etc., and achieves the reduction of crystallinity, The effect of lowering glass transition temperature and improving toughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The present invention also provides a method for preparing the 2,5-thiophenedicarboxylate copolyester described in the above technical solution, comprising the following steps:
[0060] a) Under the action of a catalyst, the diacid and diol A are subjected to an esterification reaction to obtain a prepolymer;
[0061] The diacids are 2,5-thiophenedicarboxylic acid and terephthalic acid;
[0062] or
[0063] Under the action of a catalyst, the diester and diol B are subjected to a transesterification reaction to obtain a prepolymer;
[0064] The diester is 2,5-thiophenedicarboxylic acid diester and terephthalic acid diester;
[0065] b) subjecting the prepolymer to polycondensation reaction to obtain 2,5 thiophenedicarboxylic acid-based copolyester;
[0066] The diol A and diol B are independently selected from C3-C20 linear diols, 1,4-cyclohexanedimethanol, 1,4-cyclohexanediol, isosorbide, 2,5-furan One or more of dimethanol and diethylene glycol.
[0067] In the p...
Embodiment 1
[0089] Under the action of ethylene glycol antimony, 2,5-thiophenedicarboxylic acid, terephthalic acid and 1,3-propanediol were added to the reaction flask, wherein the mass ratio of ethylene glycol antimony to the total diacid was 0.3%, The molar ratio of 1,3-propanediol to total diacids is 2.5:1, and the molar ratio of 2,5-thiophenedicarboxylic acid to terephthalic acid is 0.24:0.80. Under the protection of nitrogen, the reaction was stirred at 200° C. for 3 h to obtain a prepolymer. The above prepolymer was evacuated to 90Pa, stirred and reacted at 235°C for 5h to obtain copolyester.
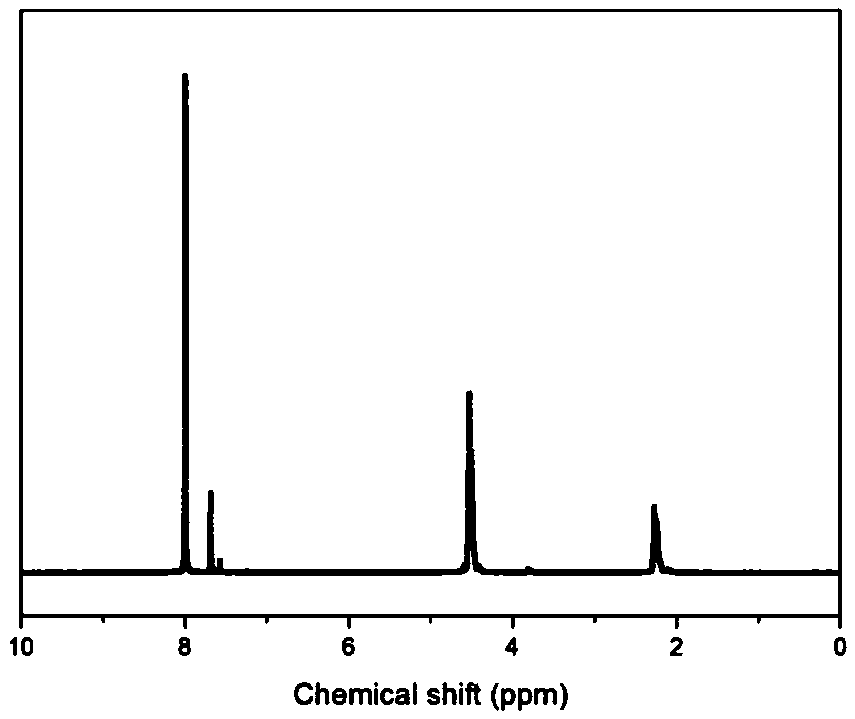
[0090] Gained copolyester is carried out proton nuclear magnetic spectrum test, the result is as follows: figure 1 as shown, figure 1 It is the H NMR spectrum of the copolyester obtained in Example 1 of the present invention. It can be seen that the resulting copolyester has a structure of formula (1), wherein R is -(CH 2 ) 3 -.
Embodiment 2
[0092] Under the action of stannous octoate, dimethyl 2,5-thiophenedicarboxylate, dimethyl terephthalate and 1,4-butanediol were added to the reaction flask, wherein stannous octoate accounted for 1% of the total diester The mass ratio is 0.3%, the molar ratio of 1,4-butanediol to total diester is 3:1, and the molar ratio of dimethyl 2,5-thiophenedicarboxylate to dimethyl terephthalate is 0.23:0.77 . Under the protection of nitrogen, the reaction was stirred at 200° C. for 3 h to obtain a prepolymer. The above prepolymer was evacuated to 80Pa, stirred and reacted at 235°C for 8h to obtain copolyester.
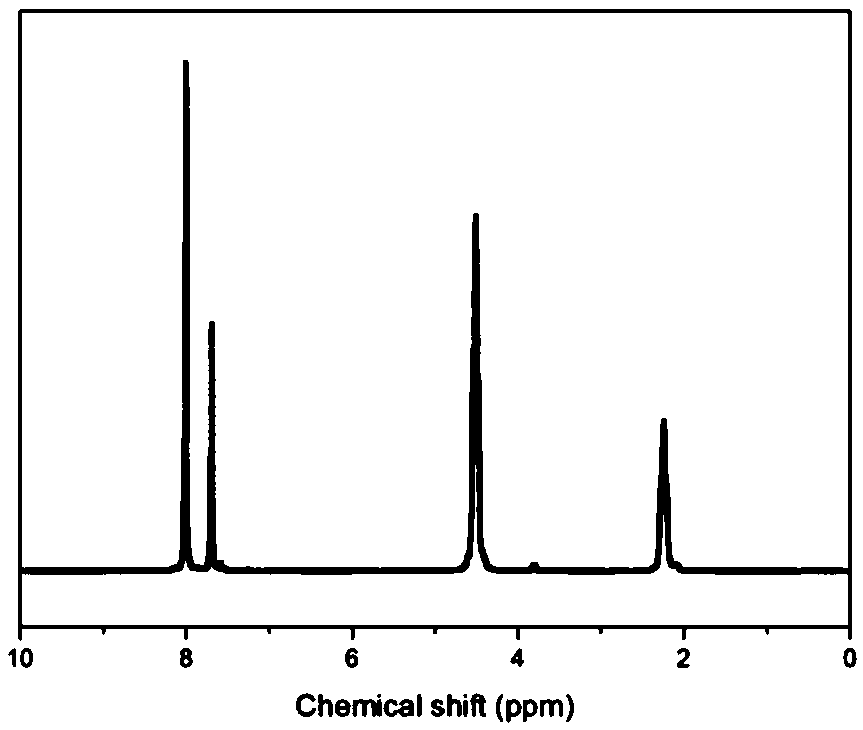
[0093] Gained copolyester is carried out proton nuclear magnetic spectrum test, the result is as follows: figure 2 as shown, figure 2 It is the H NMR spectrum of the copolyester obtained in Example 2 of the present invention. It can be seen that the resulting copolyester has a structure of formula (1), wherein R is -(CH 2 ) 4 -.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com