Ternary composite metal oxide solid alkali catalyst and preparation method and application thereof
A solid base catalyst, ternary composite technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of low catalyst activity, easy agglomeration and deactivation, Problems such as low yield of glycerol carbonate, to achieve the effect of high specific surface area, rich in basic sites, inhibition of growth and aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
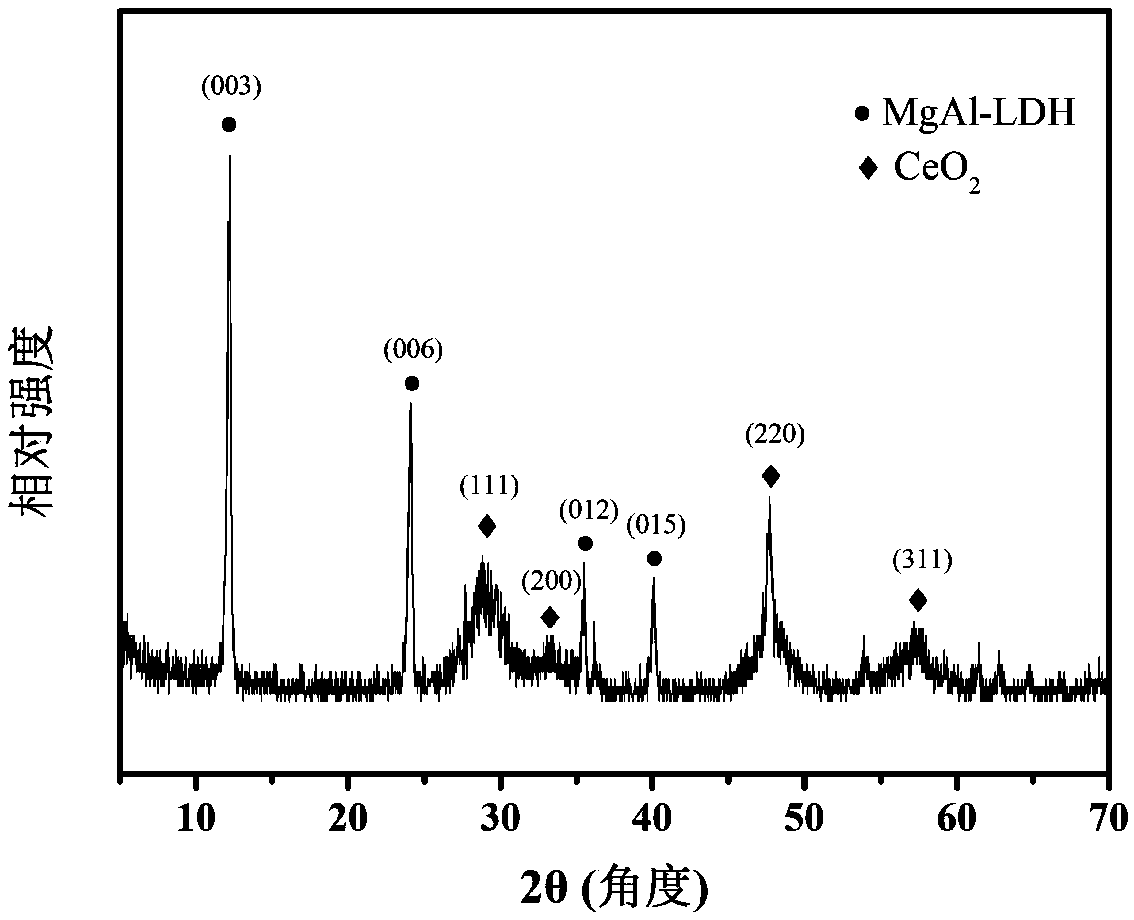
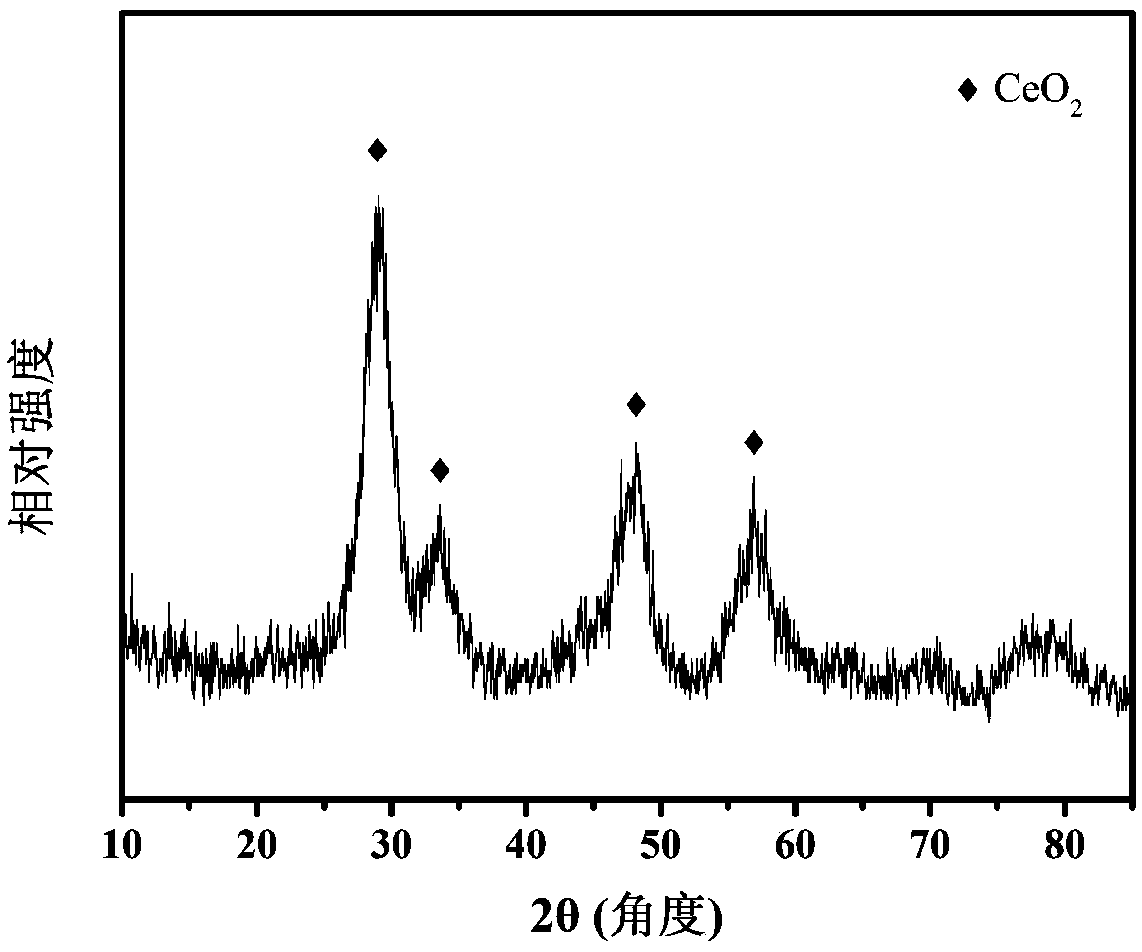
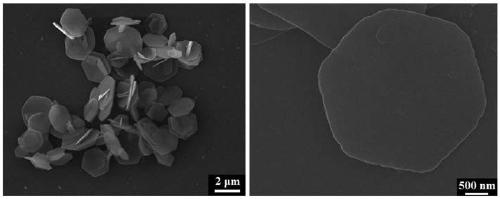
[0039] A. Accurately weigh 5.1282g Mg(NO 3 ) 2 ·6H 2 O, 3.7519g Al(NO 3 ) 3 9H 2 O was mixed with deionized water to prepare 40 mL of mixed metal salt solution. Accurately weigh 1.8018g of urea, prepare 40mL of urea solution, and mix it evenly with metal salt solution, then put it in a reaction kettle for hydrothermal crystallization at 120°C for 24h, centrifuge, wash until neutral, and place at 70°C drying to obtain the MgAl-LDH precursor material.
[0040] Accurately weigh 0.5046g Ce(NO 3 ) 3 ·6H 2 Prepare 40 mL of cerium salt solution with O water; accurately weigh 1 g of the MgAl-LDH precursor in step A and disperse in the cerium salt solution, and ultrasonically treat for 10 min to form a stable cerium salt suspension; The sodium borohydride solution, and the cerium salt suspension were added to the colloid mill and stirred for 5 minutes, then placed in a hydrothermal kettle equipped with a polytetrafluoroethylene liner, heated to 150°C in an oven for hydrotherma...
Embodiment 2
[0044] Accurately weigh 10.2564g Mg(NO 3 ) 2 ·6H 2 O, 3.7519g Al(NO 3 ) 3 9H 2 O was mixed with deionized water to prepare 40 mL of mixed metal salt solution. Accurately weigh 3.0030g of urea, prepare 40mL of urea solution, and mix it with metal salt solution evenly, then put it in a reaction kettle for hydrothermal crystallization at 120°C for 24h, centrifuge, wash until neutral, and store at 70°C drying to obtain the MgAl-LDH precursor material.
[0045] Accurately weigh 0.5046g Ce(NO 3 ) 3 ·6H 2 Prepare 40 mL of cerium salt solution with O water; accurately weigh 1 g of the MgAl-LDH precursor in step A and disperse in the cerium salt solution, and ultrasonically treat for 10 min to form a stable cerium salt suspension; The sodium borohydride solution, and the cerium salt suspension were added to the colloid mill and stirred for 5 minutes, then placed in a hydrothermal kettle equipped with a polytetrafluoroethylene liner, heated to 150°C in an oven for hydrothermal ...
Embodiment 3
[0049]Accurately weigh 4.066g MgCl 2 ·6H 2 O, 2.4143g AlCl 3 ·6H 2 O was mixed with deionized water to prepare 40 mL of mixed metal salt solution. Accurately weigh 1.8018g of urea, prepare 40mL of urea solution, and mix it evenly with metal salt solution, then put it in a reaction kettle for hydrothermal crystallization at 120°C for 24h, centrifuge, wash until neutral, and place at 70°C drying to obtain the MgAl-LDH precursor material.
[0050] Accurately weigh 0.4255g CeCl 3 ·7H 2 Prepare 40 mL of cerium salt solution with O water; accurately weigh 1 g of the MgAl-LDH precursor in step A and disperse in the cerium salt solution, and ultrasonically treat for 10 min to form a stable cerium salt suspension; The sodium borohydride solution and the cerium salt suspension were added to the colloid mill and stirred for 5 minutes, then placed in a hydrothermal kettle equipped with a polytetrafluoroethylene liner, heated to 120°C in an oven for hydrothermal crystallization for 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com