Heterologous expression purification method for recombination high temperature nickel and iron hydrogenase and application thereof
A nickel-iron hydrogenase and heterologous expression technology, which is applied in the field of expression and purification of nickel-iron hydrogenase and coenzyme regeneration, can solve the problems of regeneration that have not been studied yet, and achieve the effect of low production cost, high enzyme yield and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
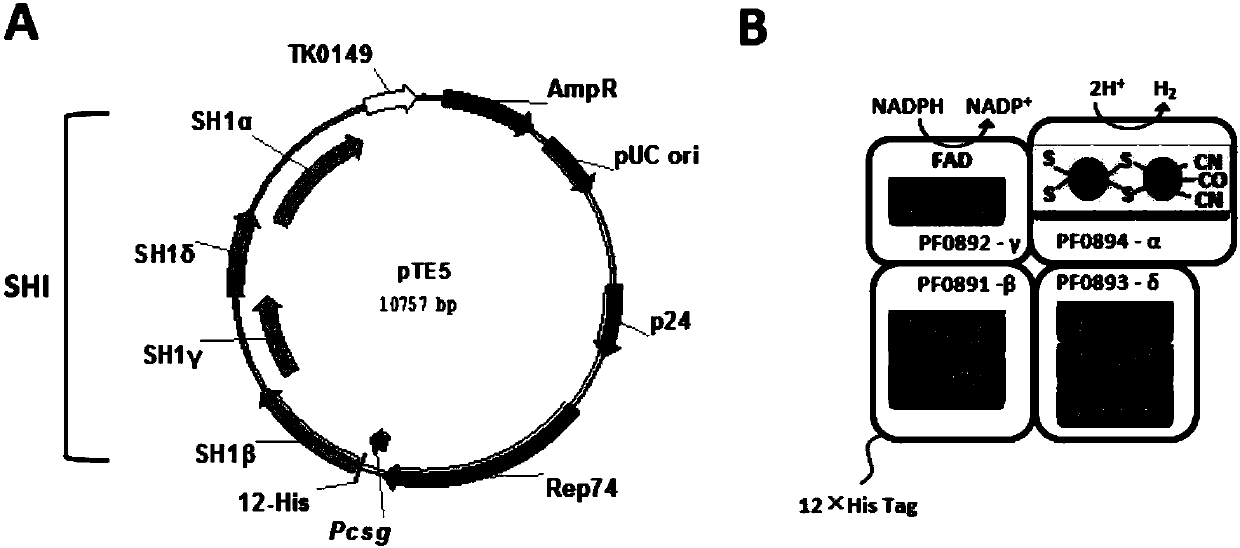
[0062] Experimental example 1 T.kodakarensis shuttle vector construction
[0063] The present invention utilizes a shuttle vector to overexpress SHI in T. kodakarensis.
[0064] Using pTS543 as a template (plasmid derived from reference Santangelo TJ, Lu, Reeve JN. 2010. Thermococcus kodakarensis genetics: TK1827-encoded β-glycosidase, newpositive-selection protocol, and targeted and repetitive deletion technology. Appl. Environ. Microbiol. 76(4): 1044-52.), with primer 1 and primer 2 Amplify the target fragment, which contains all the sequences of the plasmid pTN1 autonomously replicating in T.kodakarensis and the genetic elements expressing the screening marker gene TK0149, the primer 1 sequence is:
[0065] GAAGCTCAGGTGGTACTTCACTCCACAATGGTTTCTTAGACGTCAGGTGGC, primer 2 sequence is:
[0066] GAATTTGCCAAATTGCCAGAATTGGCCATAGCTGTTTCCTGTGTGAAATTG; In addition, using pUC19 as a template, use primer 3 and primer 4 to amplify its origin of replication and the sequence of expressi...
experiment example 2
[0069] Experimental example 2 Construction of expression vector of recombinant high-temperature nickel-iron hydrogenase SHI
[0070] Access to Strong Promoter P via NCBI csg Sequence, referenced as (Kanai T, Simons JR, Tsukamoto R, Nakajima A, Omori Y, Matsuoka R, Beppu H, Imanaka T, Atomi H.2015.Overproduction of the membrane-bound[NiFe]-hydrogenase in Thermococcus kodakarensis and its effect on hydrogen production.Front.Microbiol.6:847.), design primer 5 and primer 6 (primer 5:
[0071] GAATTCTGCAGATATCCATCACACTGCGGCAAAAGGCGAATTATGTGTAG, Primer 6:
[0072] P csg Fragment; In addition, using pTE1 in Experimental Example 1 as a template, design primer 7
[0073] (CCCCAACAACCCAAGGAGGTGTTGTGCGGCCGCAAAAAAGTCGACTGCCG) and primer 8
[0074] (CTACACATAATTCGCCTTTTGCCGCAGTGTGATGGATATCTGCAGAATTC) amplified plasmid backbone; then by Simple Cloning (You, C., et al. (2012). "Simple Cloning via DirectTransformation of PCR Product (DNA Multimer) to Escherichia coli and Bacillus subtilis...
experiment example 3
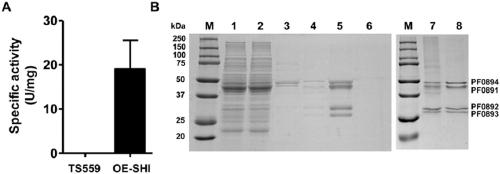
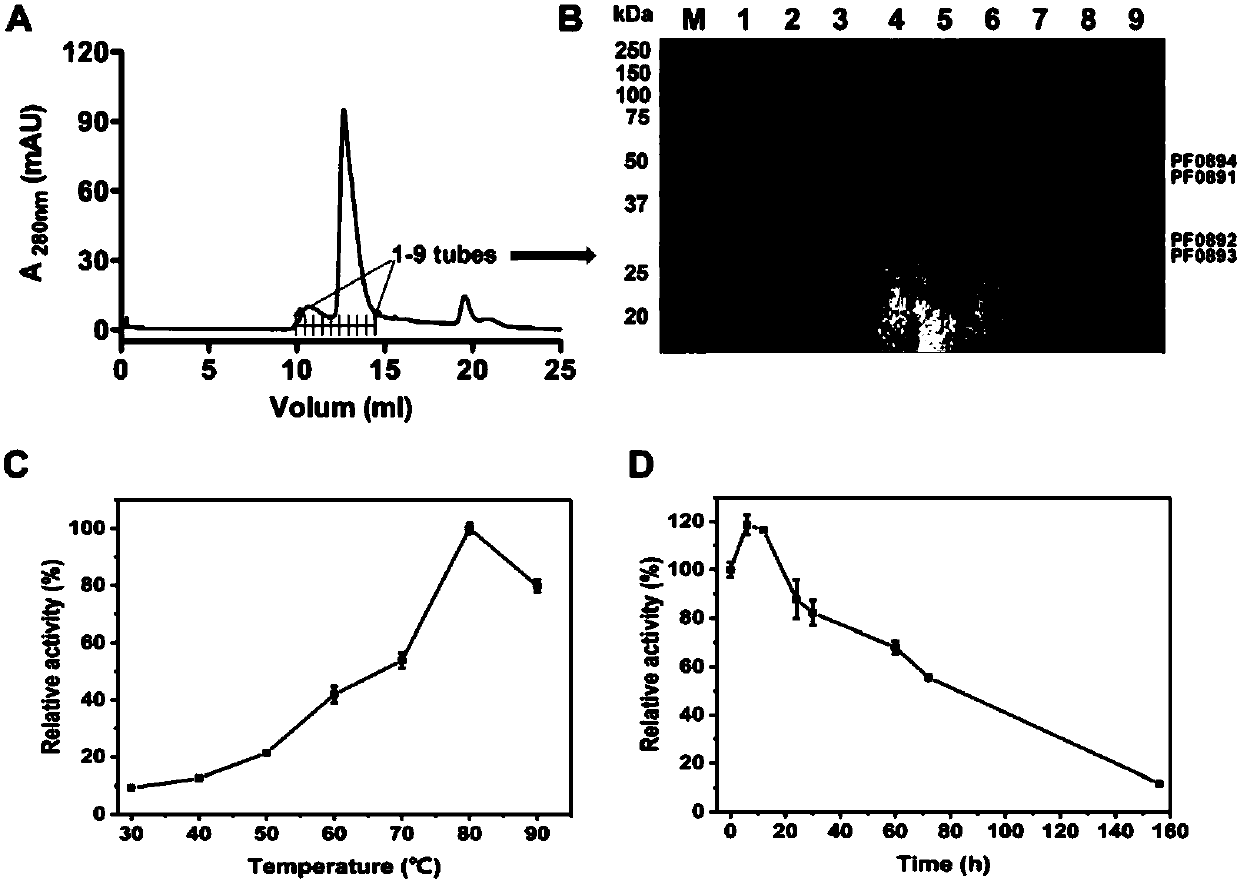
[0085] Experimental Example 3 Detection of Hydrogenase Enzyme Activity
[0086] The hydrogenase detection method based on benzyl viologen BV used in the present invention is as follows: add 2ml reaction mixture (1mM BV, 100mM EPPS, pH 8.4) in 3ml sealed cuvette, fill in the anaerobic bottle containing 3% A mixed gas of hydrogen (nitrogen:hydrogen=97:3). After preheating the reaction solution and the enzyme sample at 85°C, add the enzyme to start the reaction. The reaction was carried out in a temperature-controllable 100CaryUV-Vis spectrophotometer (Agilent), the temperature was maintained at 85°C, and the change in absorbance at 578nm was detected in real time, that is, the amount of reduced BV produced. 1 U of enzyme activity is equivalent to oxidizing 1 μmol of hydrogen or reducing 2 μmol of BV in 1 min.
[0087] For the method of detecting hydrogenase with other electron carriers (NADP or methyl viologen MV, etc.), refer to the above detection method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com