Near-infrared bio-thiol fluorescent probe as well as preparation method and application thereof
A thiol and reaction technology, which is applied in the field of near-infrared biothiol fluorescent probes and its preparation, can solve problems affecting the detection effect and achieve broad application prospects, easy control of the reaction, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The synthetic route of the target compound B of this embodiment is as follows:
[0058]
[0059] Specifically include the following steps:
[0060] (1) Add 276 mg of isophorone (2 mmol) and 330 mg of malononitrile (5 mmol) dropwise into 30 mL of absolute ethanol, add 3 drops of piperidine dropwise under magnetic stirring, heat to reflux for 12 hours, cool to room temperature, and precipitate a solid , the filter cake was collected by suction filtration, and the filter cake was recrystallized with absolute ethanol to obtain light yellow flaky solid intermediate I with a yield of 78%.
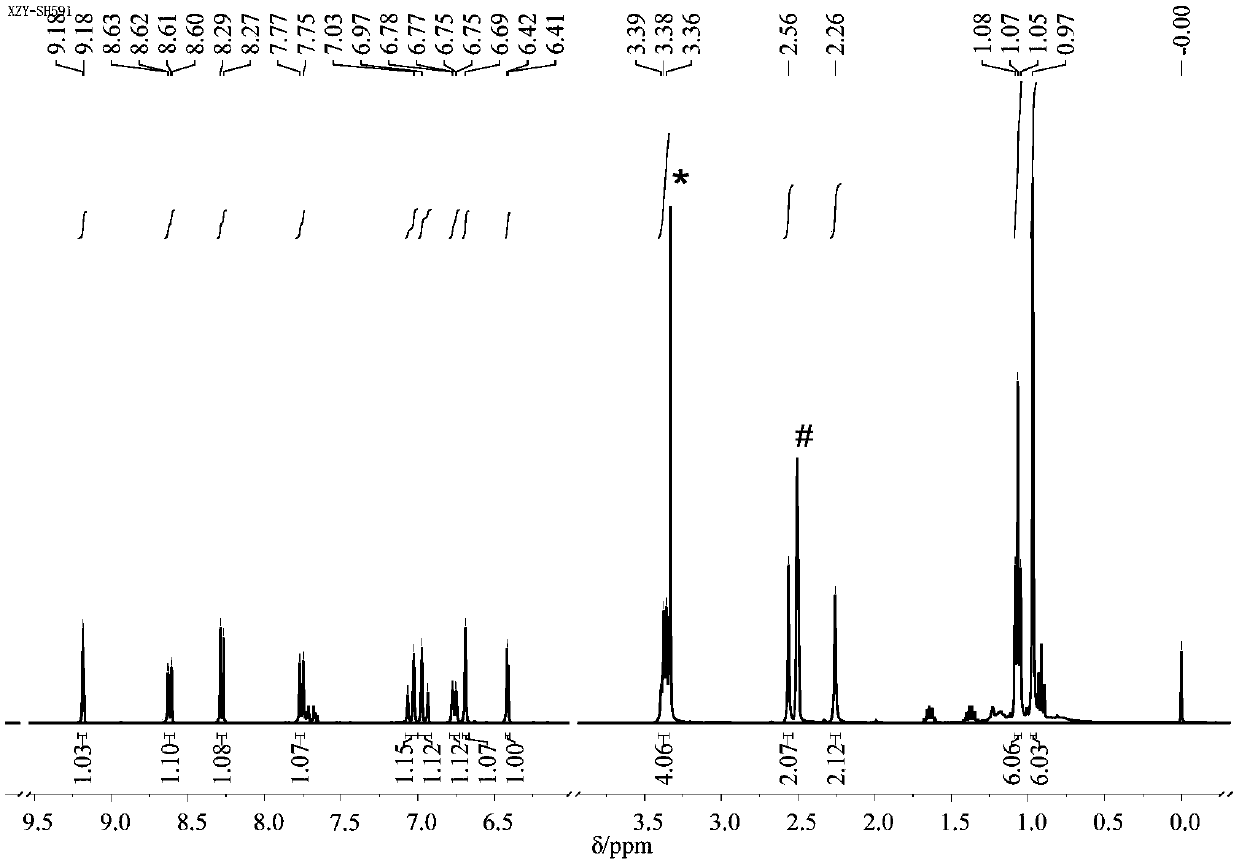
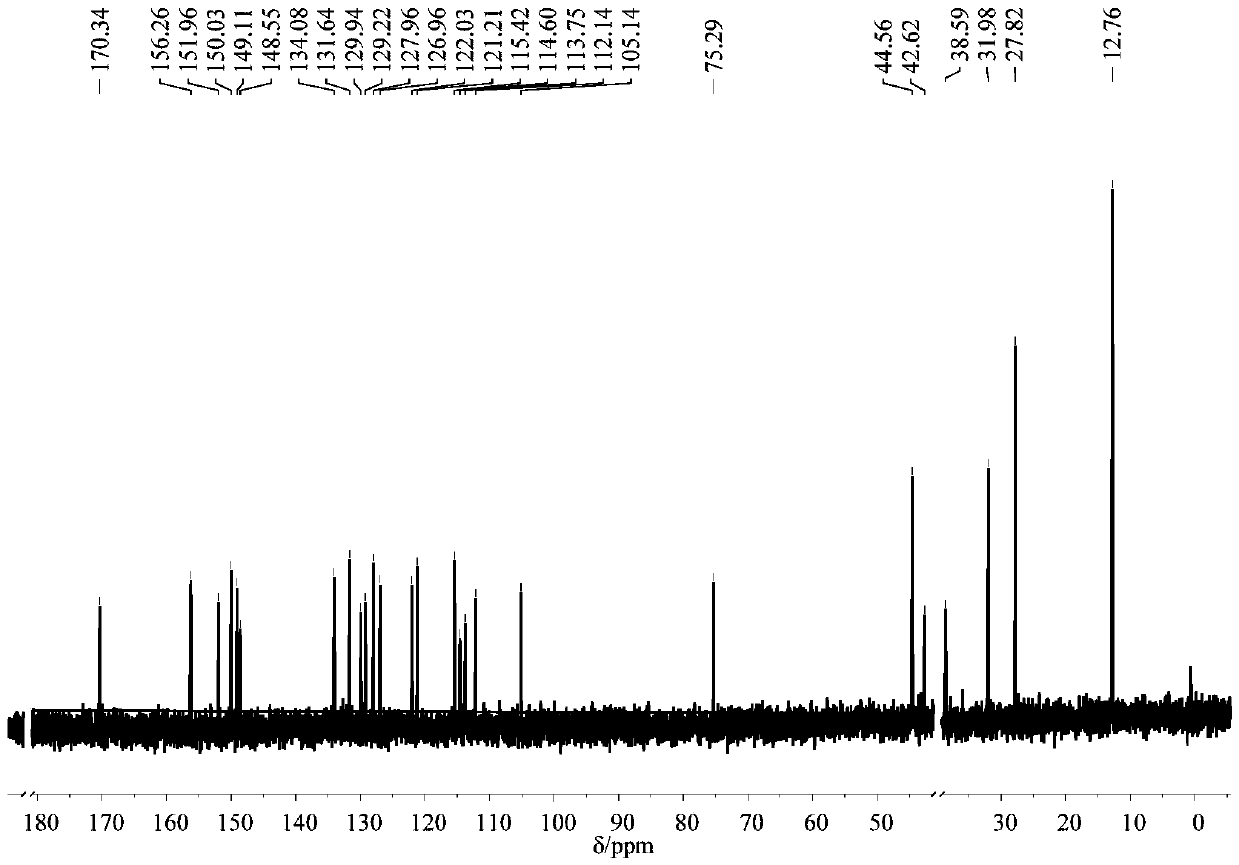
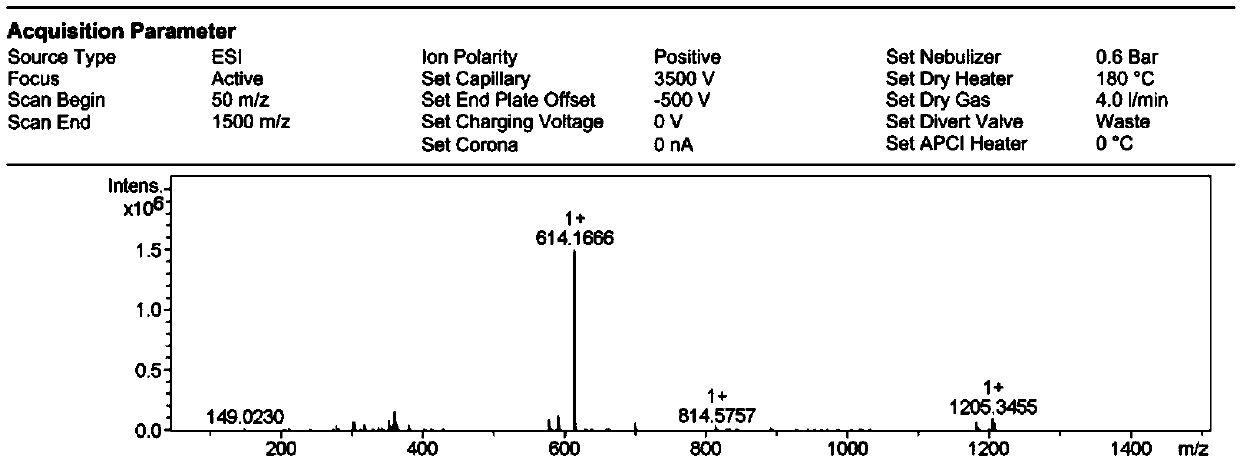
[0061] (2) Dissolve 186mg of intermediate I (1mmol) into 15mL of absolute ethanol, add 193mg of 4-(N,N-diethyl) salicylaldehyde (1mmol), and add 3 drops of piperaldehyde under magnetic stirring Pyridine was heated to reflux for 4 h, cooled to room temperature, and the solvent was removed by rotary evaporation to obtain a residue. The residue was subjected to column chromatography to ob...
Embodiment 2
[0069] The synthetic route of the target compound B of this embodiment is the same as that of Example 1.
[0070] Specifically include the following steps:
[0071] (1) 276mg of isophorone (2mmol) and 330mg of malononitrile (5mmol) were added dropwise in 20mL of acetonitrile, under magnetic stirring, 3 drops of piperidine were added dropwise, heated to reflux for 8h, cooled to room temperature, precipitated solid, pumped The filter cake was collected by filtration, and the filter cake was recrystallized with absolute ethanol to obtain light yellow flaky solid intermediate I with a yield of 89%.
[0072] (2) Dissolve 186 mg of intermediate I (1 mmol) into 15 mL of acetonitrile, add 193 mg of 4-(N,N-diethyl) salicylaldehyde (1 mmol), and add 3 drops of piperidine dropwise under magnetic stirring, Heated to reflux for 6 h, cooled to room temperature, and the solvent was removed by rotary evaporation to obtain a residue, which was subjected to column chromatography to obtain Comp...
Embodiment 3
[0076] In this example, the target compound B prepared in Example 1 was used as a probe to detect different concentrations of biothiols.
[0077] The target compound B prepared in Example 1 was dissolved in dimethyl sulfoxide to prepare a stock solution with a concentration of 10 mM. Take 3 μL of the stock solution and place it in a 5 mL centrifuge tube, add different concentrations of Cys, then adjust the total volume to 3 mL with ethanol-PBS (v:v=1:1, pH=7.4, 10 mM), mix well and place at room temperature After 10 minutes, a series of test solutions with different concentrations of Cys were obtained. Wherein, adding different concentrations of Cys to the test solution, the concentrations of Cys are: 0 μM, 1 μM, 2 μM, 3 μM, 4 μM, 5 μM, 6 μM, 7 μM.
[0078] Adopt ultraviolet spectrophotometer to measure ultraviolet absorption spectrum, the result is as follows Figure 4 shown. From Figure 4 It can be seen that with the increase of the concentration of Cys, the maximum abs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com