Preparation method of methylprednisolone hemisuccinate impurity
A technology of methylprednisolone succinate and succinate, which is applied in the field of chemical synthesis to achieve the effects of improving quality standards, clear synthesis paths and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
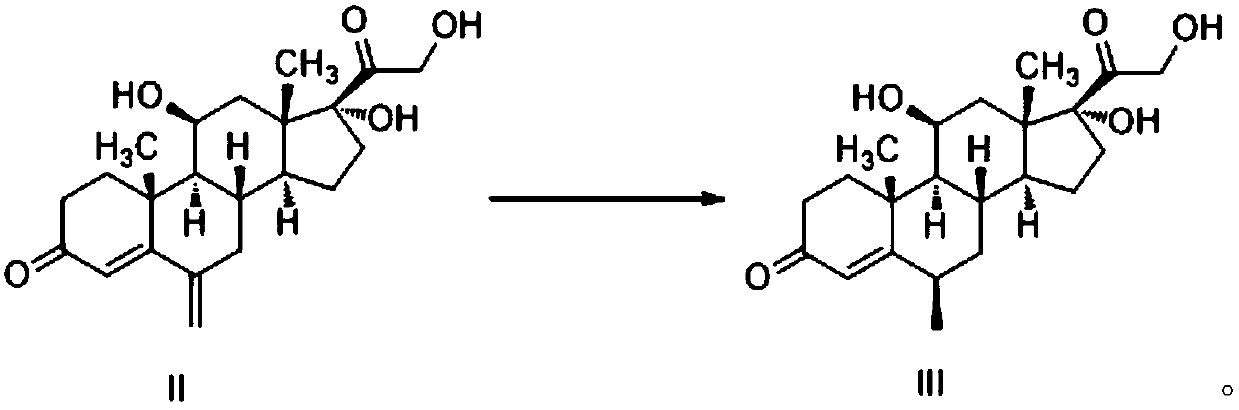
[0042] Step 1: Preparation of 11β, 17α, 21-trihydroxy-6β-methylpregna-4-ene-3,20-dione (Formula III)
[0043] to N 2 Add 10.0g 11β, 17α, 21-trihydroxy-6-methylenepregn-4-ene-3,20-dione, 250ml absolute ethanol and 50ml cyclohexene into the protected reaction flask, stir, then add 0.68g of triethylamine and 4.0g of 10% Pd / C, heated up to 65-70°C. Insulate and react at 65-70°C for 3h. The reaction solution was filtered to remove the catalyst Pd / C, and the filtrate was evaporated to dryness under reduced pressure to obtain 9.4 g of off-white solid with a yield of 93.5% and a 6β-isomer content of 94.7%.
[0044] Step 2: Preparation of 11β, 17α-dihydroxy-6β-methylpregna-4-ene-3,20-dione-21-acetate (Formula IV)
[0045] to N 2 Add 9.0g11β, 17α, 21-trihydroxy-6β-methylpregn-4-ene-3,20-dione (formula III) and 135ml dichloromethane into the protected reaction flask, stir, then add 3.6g three Ethylamine and 2.93g of acetic anhydride were added and kept warm at 25°C until the end of ...
Embodiment 2
[0056] Preparation of 11β, 17a, 21-trihydroxy-6β-methylpregn-4-ene-3,20-dione (Formula III)
[0057] to N 2 Add 10.0g 11β, 17a, 21-trihydroxy-6-methylenepregn-4-ene-3,20-dione, 250ml absolute ethanol and 50ml cyclohexene into the protected reaction flask, stir, then add 0.68g of triethylamine and 4.0g of 10% Pd / C, heated up to 65-70°C. Insulate and react at 65-70°C for 3h. The reaction solution was filtered to remove the catalyst Pd / C, and the filtrate was evaporated to dryness under reduced pressure to obtain 9.4 g of off-white solid with a yield of 93.5% and a 6β-isomer content of 94.7%.
Embodiment 3
[0059] Preparation of 11β, 17α-dihydroxy-6β-methylpregna-4-ene-3,20-dione-21-acetate (Formula IV)
[0060] to N 2 Add 9.0g11β, 17α, 21-trihydroxy-6β-methylpregn-4-ene-3,20-dione (formula III) and 135ml dichloromethane into the protected reaction flask, stir, then add 3.6g three Ethylamine and 2.93g of acetic anhydride were added and kept warm at 25°C until the end of the reaction. The reaction system was poured into 180ml of ice water, stirred, left to stand for liquid separation, the water phase was extracted twice with 200ml of dichloromethane, and the organic phase was combined. Washed three times with 200 ml of saturated brine; the organic phase was evaporated to dryness under reduced pressure to obtain 9.8 g of light yellow solid with a yield of 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com