Universal-type CAR-T cell based on base editing and preparation method and application thereof
A general-purpose, cell-based technology, applied in the field of preparation of general-purpose CAR-T cells, can solve problems such as complex preparation process, off-target effects, and immune system damage, and achieve simplified preparation procedures, improved transfection efficiency, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Construction of recombinant expression vectors.
[0057] The single base editing system used in the present invention is BE-Plus: a stop codon is introduced through CT mutation to achieve gene knockout. BE-Plus two-plasmid system (scFv-APOBEC-UGI, GCN4-D10A), 10 GCN4s are connected to the N-terminal of D10A, and one scFv is connected to the N-terminal of APOBEC. In this way, GCN4-D10A can recruit 10 scFv-APOBECs, increasing the probability of mutation. The work window is expanded from 4-8 to 4-16, making the efficiency higher.
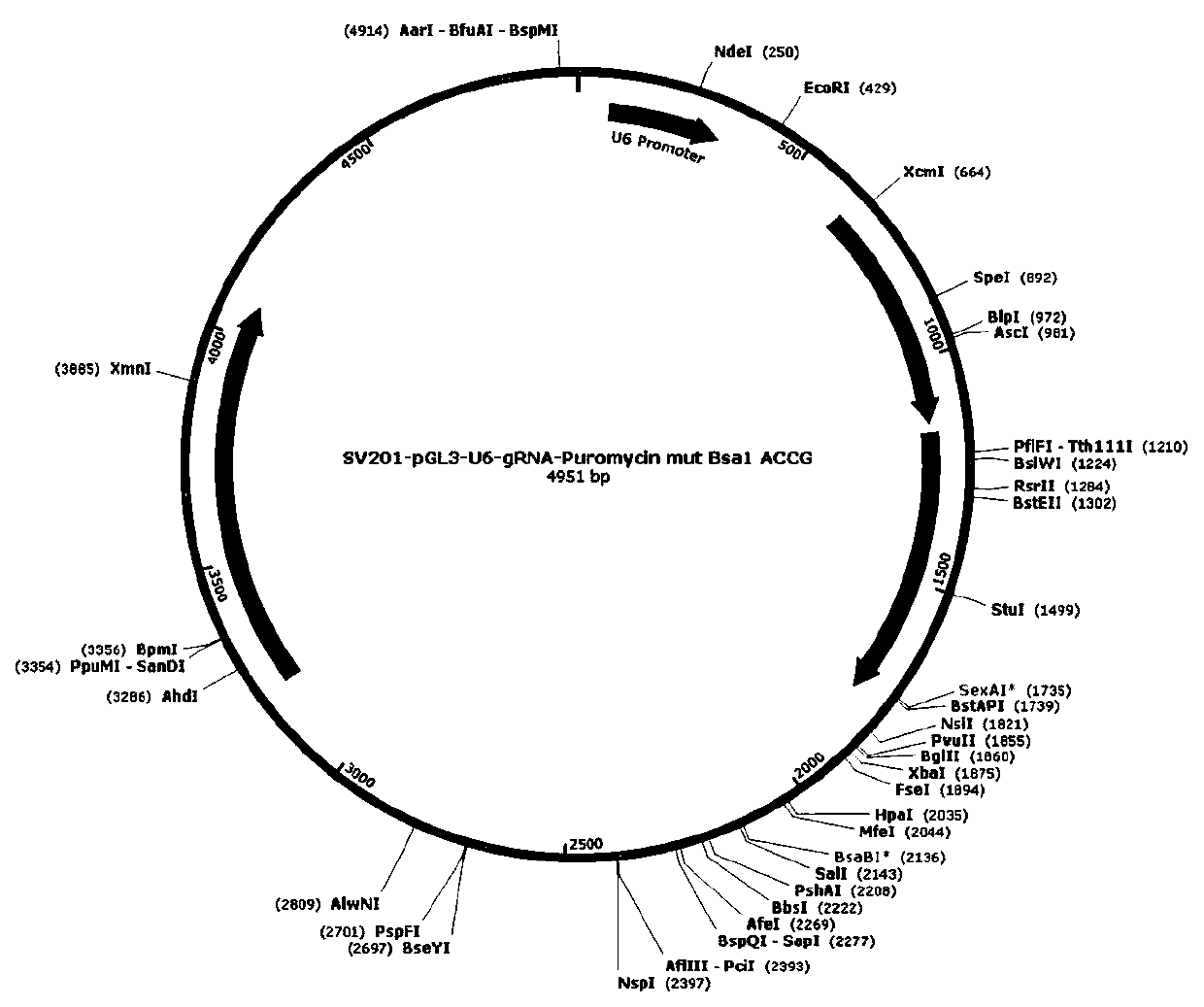
[0058] Use SV201-pGL3-U6-gRNA-Puromycin mut Bsa1 ACCG in the present invention (see figure 1 ) as a backbone carrier, linearized by restriction endonuclease Bsa1 (NEB), and ligated with artificially synthesized sgRNA under the action of T4 ligase to form a recombinant vector.
[0059] Synthesis of TRAC-sgRNA and B2M-sgRNA: Select sgRNA as reverse sgRNA (CCA is the target codon, GGG is the PAM sequence). TRAC-sgRNA synthesized unidi...
Embodiment 2
[0060] Example 2 sgRNA functional verification.
[0061] 1) Cell culture and transfection.
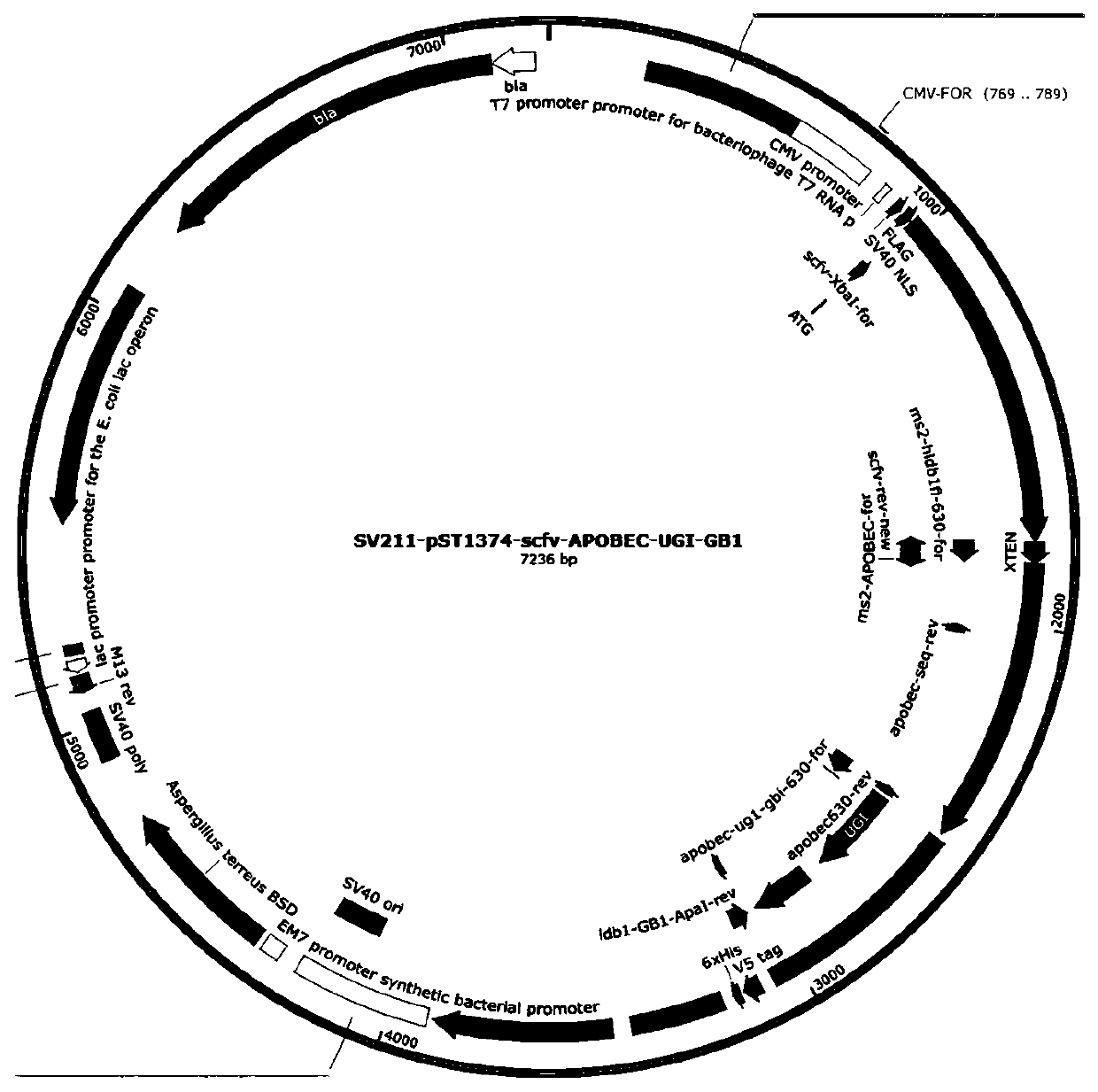
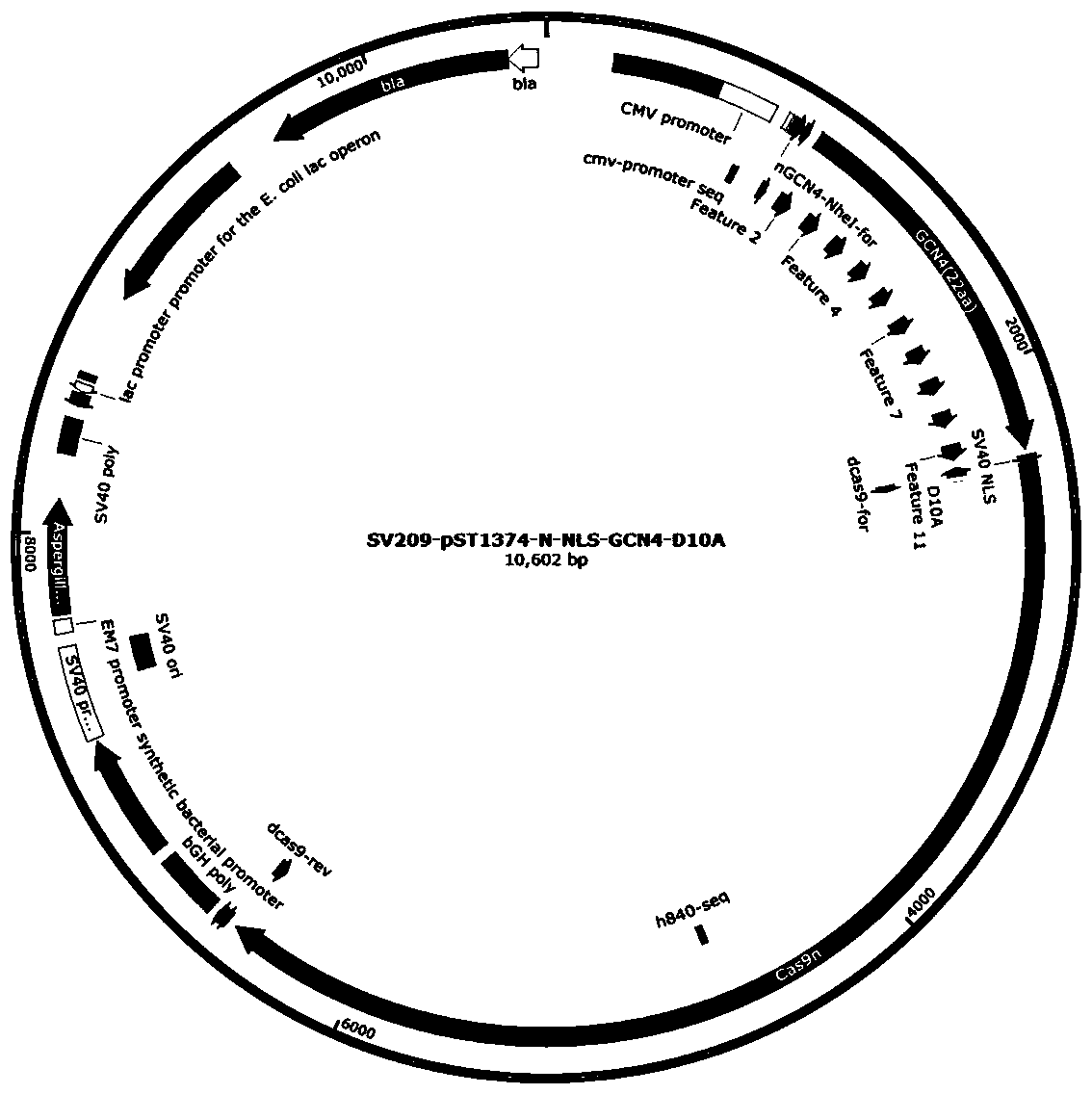
[0062]A. Use the electroporation kit to transfect HEK293T cells to verify the targeting efficiency of sgRNA. 2 µg pST1374-scFv-APOBEC-UGI-GB1 (see figure 2 ), 2.0 µg of pST1374-N-NLS-GCN4-D10A (see image 3 ), 2µgpGL3-U6-TRAC sgRNA BE plasmid was mixed with the electroporation solution, and the cells were co-transfected. After 6-8 hours, the medium was changed, and Blasticidin (invitrigen, ant-bl-1) and Puromycin (invitrigen, ant-pr-1) and Puromycin (invitrigen, ant-pr- 1) Drug screening, collect cells after 48 hours.
[0063] B. Use the electroporation kit to transfect HEK293T cells to verify the targeting efficiency of sgRNA, add 2 µg pST1374-scFv-APOBEC-UGI-GB1 (see figure 2 ), 2.0 µg of pST1374-N-NLS-GCN4-D10A (see image 3 ), 2µgpGL3-U6-B2M sgRNA BE plasmid was mixed with the electroporation solution, and the cells were co-transfected. After 6-8 hours, the medium was changed...
Embodiment 3
[0067] Example 3 Preparation of PBMC cells and sorting of CD3 positive T cells.
[0068] 1) Use a blood collection bag to collect 80-100 mL of peripheral blood from healthy people (shake while collecting to fully mix the peripheral blood with the anticoagulant), and isolate PBMCs in a sterile cell preparation workshop. Transfer the peripheral blood to a 50ml centrifuge tube, centrifuge at 800g at room temperature for 15 minutes, absorb the upper layer of yellow autologous plasma into a new 50ml centrifuge tube, inactivate at 56°C for 30 minutes, and store it at -20°C for later use; add to the lower cell layer Equal volume of PBS, mix up and down;
[0069] 2) Draw 15ml of lymphocyte separation medium (Ficoll-Paque Plus, GE) at the bottom of a new 50ml centrifuge tube, and slowly add 30ml of diluted blood along the tube wall to the upper layer of the lymphocyte separation medium, put the centrifuge tube in a centrifuge, 450g, slowly Rise and descend slowly for 25 minutes, no br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com