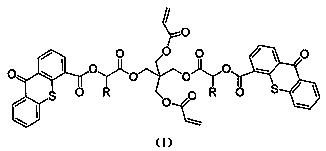
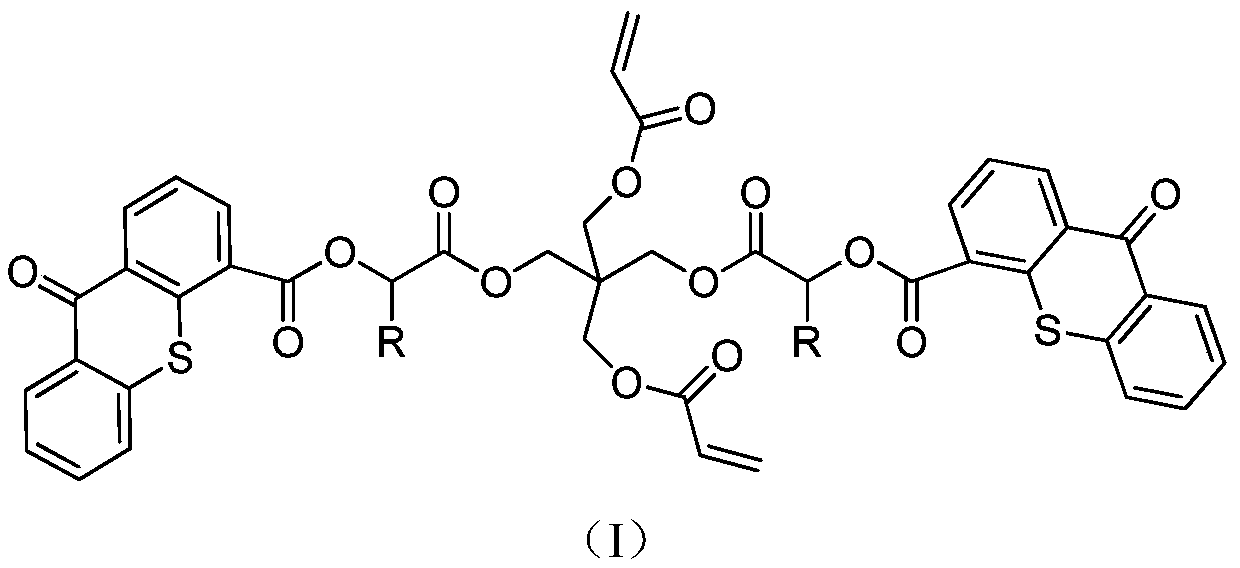
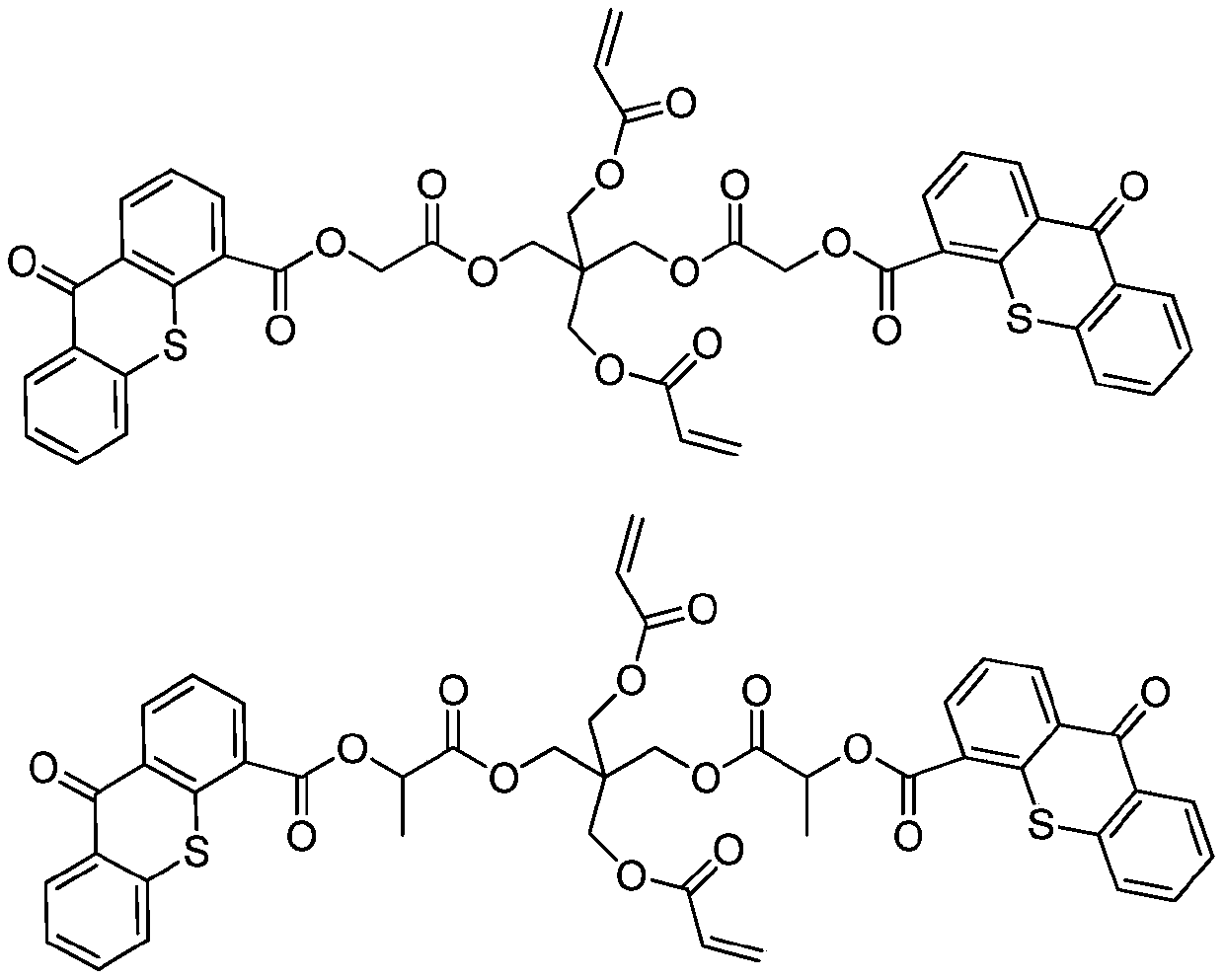
Polymerizable II-type photoinitiator and preparation method thereof
A technology for polymerizing photoinitiators and compounds, which is applied in the field of polymerizable type II photoinitiators and their preparation, can solve the problems of toxicity and deterioration of physical properties of packaging materials, and achieve low mobility, excellent photocuring performance, and high activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of Compound A
[0032] Add 48.8g of pentaerythritol diacrylate (0.2mol), 23.3g of triethylamine (0.21mol) and 240ml of dichloromethane into the three-necked flask, and react with electromagnetic stirring. A constant pressure dropping funnel with 46.3g of chloroacetyl chloride (0.41mol) and 120ml of dichloromethane is slowly added dropwise at low temperature, and the temperature is kept between 0 and -5°C. After the dropwise addition is completed, move to room temperature for reaction. TLC monitored the completion of the reaction. Filter triethylamine hydrochloride, wash with saturated sodium bicarbonate 2-3 times, then wash with saturated brine, dry over anhydrous magnesium sulfate, and spin dry. Separation and purification by chromatographic column to obtain a transparent oily substance with a yield of about 85% and a purity of ≥95%. 1 H NMR (400MHz, CDCl 3 )δ6.45(dd,1H), 6.05(m,2H), 5.58(dd,2H), 4.34(s,4H), 4.10(s,4H), 4.00(s,4H).
[0033]...
Embodiment 2
[0034] Example 2: Preparation of Compound B
[0035] Add 48.8g of pentaerythritol di(acrylic acid) ester (0.2mol), 23.3g of triethylamine (0.21mol) and 240ml of dichloromethane into the three-necked flask, and react with electromagnetic stirring. The three ports of the three-necked flask are respectively connected to a thermometer and a drying tube and a constant pressure dropping funnel containing 51.6g 2-chloropropionyl chloride (0.41mol) and 120ml dichloromethane, slowly add dropwise at low temperature, the temperature is maintained between 0 and -5°C, after the dropwise addition is completed, move to React at room temperature. TLC monitored the completion of the reaction. Filter triethylamine hydrochloride, wash with saturated sodium bicarbonate 2-3 times, then wash with saturated brine, dry over anhydrous magnesium sulfate, and spin dry. Separation and purification by chromatographic column to obtain a transparent oily product with a yield of about 80% and a purity of...
Embodiment 3
[0037] Example 3: preparation of
[0038]In a 500ml four-neck flask equipped with mechanical stirring, add 50.0g thioxanthone-4-carboxylic acid, 250ml tetrahydrofuran, 23.6g triethylamine, 25.6g methyl chloroacetate, and heat to 50-60°C for 8 hours. After suction filtration, the filtrate was cooled to 0-5° C., stirred and crystallized for 2 hours, and the crude product was obtained by suction filtration. The crude product was recrystallized with toluene, and after drying, 53.7 g of yellow flaky crystals were obtained. The yield was 84%, and the content was ≥98.0%.
[0039] In a 500ml four-necked bottle equipped with a mechanical stirrer and a water separator, add 32.8g thioxanthone-4-formyloxyacetic acid methyl ester, 0.3g sodium methoxide, 200ml methylcyclohexane, 13.5g pentaerythritol diacrylic acid Esters, 0.2g p-hydroxyanisole, heated to reflux to separate methanol for 14 hours. The temperature was lowered slowly, the solid was precipitated, and filtered to obtain 24.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com