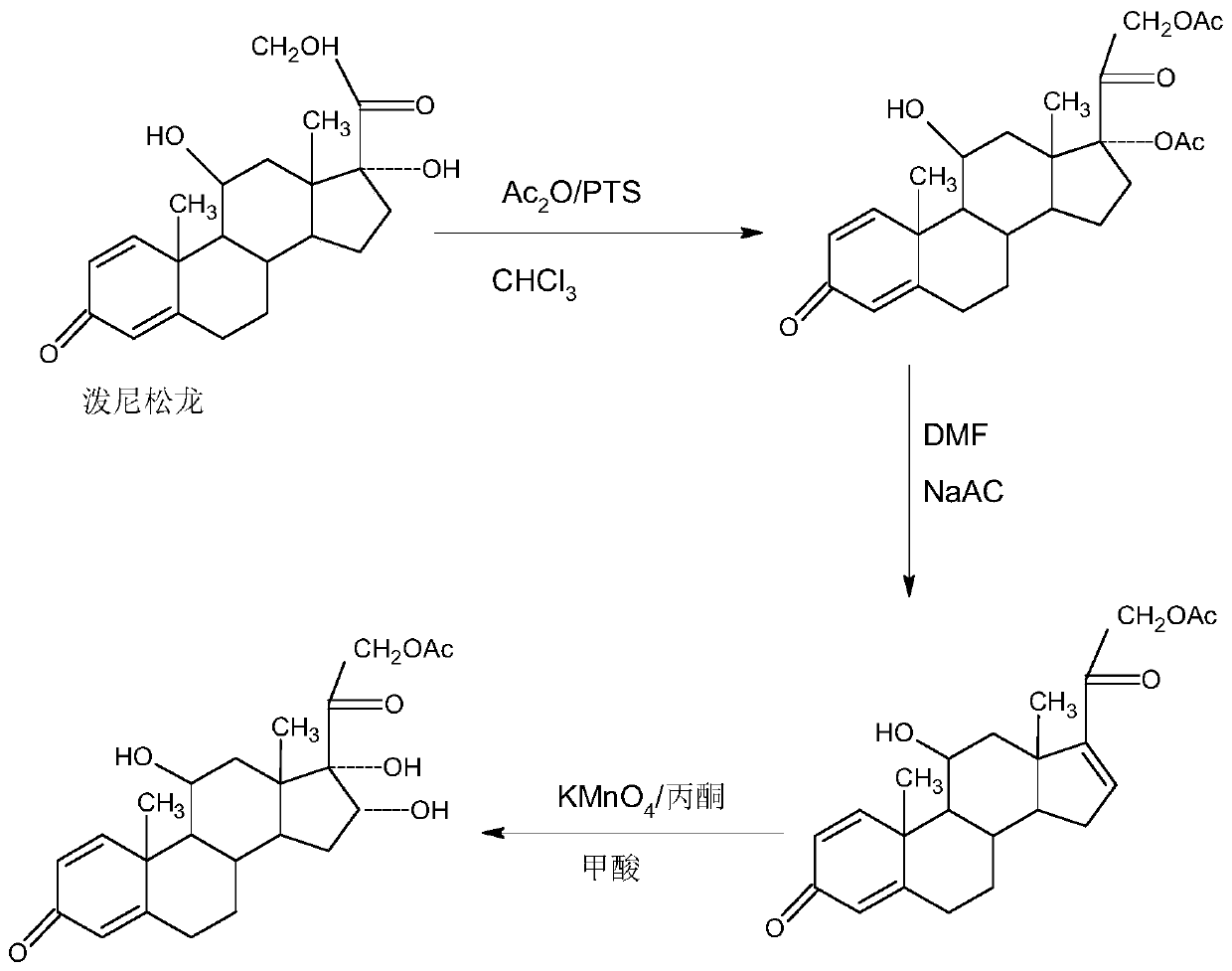
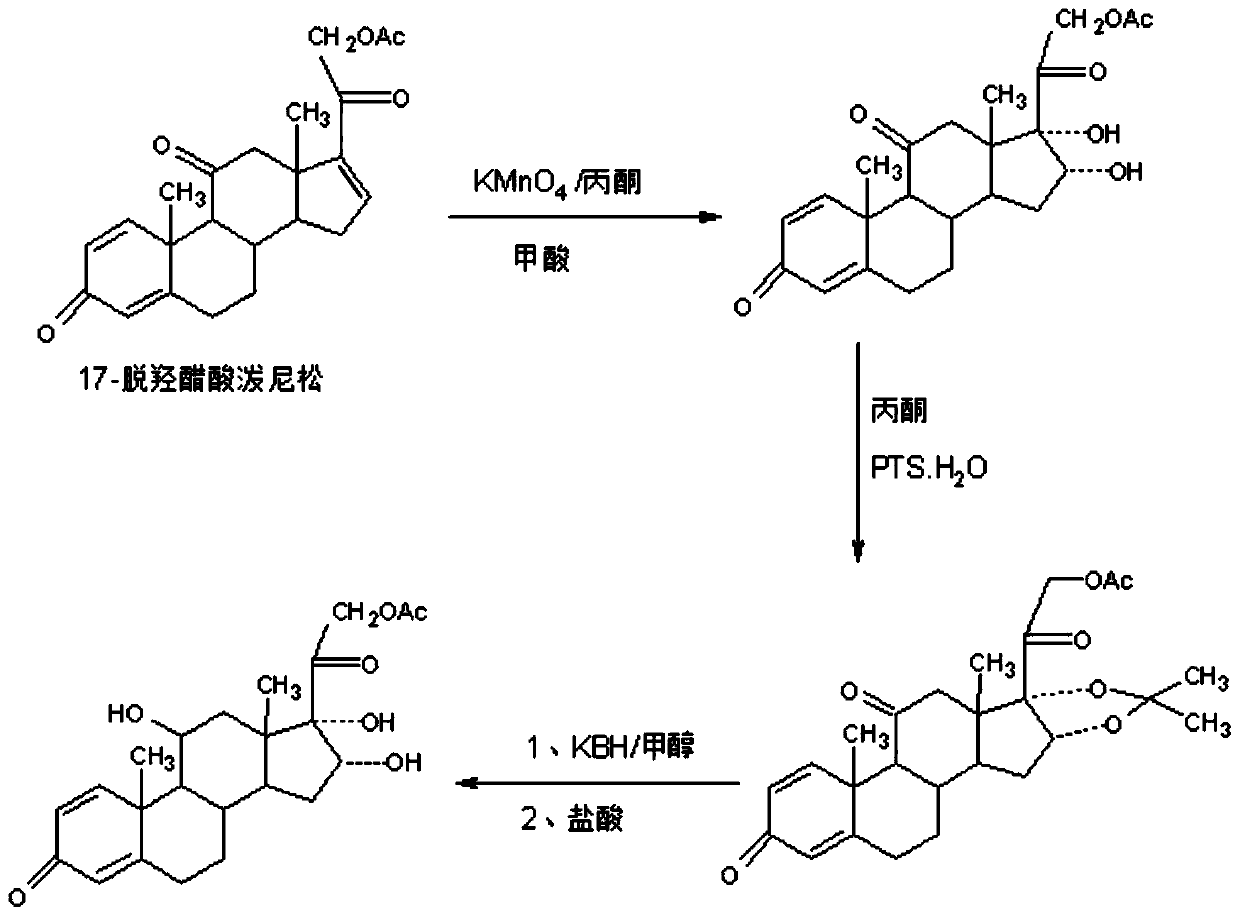
Preparation method of 16a-hydroxy prednisolone acetate product
A technology of prednisolone hydroxyacetate and prednisone hydroxyacetate, which is applied in the direction of steroids and organic chemistry, can solve the problems of high impurities, low yield, and many side reactions in oxidation reactions, and achieve high yield and low impurity Less, the effect of reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A. Preparation of oxides:
[0033] In a 5000ml three-necked flask, add 100g of 17-dehydroxyacetate prednisone, 2000ml of acetone, 12.5g of 80% formic acid, stir to dissolve the solid completely, control the temperature to 20-25°C, and add 900g of 5% dropwise within 1-1.5 hours Potassium permanganate aqueous solution, after dripping, react at 20-25°C for 1-2 hours, TLC controls the reaction end point, after the reaction, 90-95% of the solvent acetone is recovered by vacuum distillation below 35°C for the next Batch oxidation reaction, the residue was cooled to room temperature, added 1000ml of pure water for water analysis, filtered to obtain the oxide: crude product of 16a-hydroxyacetate prednisone; the crude product was recrystallized with 1000ml of 50% alcohol aqueous solution to obtain 16a-hydroxyacetate Nisone 93.8g, HPLC content 97.5%, weight yield about 93.8%;
[0034] B. Preparation of protection:
[0035] In a 1000ml three-neck flask, add 100g of the oxide pre...
Embodiment 2
[0042] A. Preparation of oxides:
[0043] In a 5000ml three-necked flask, add 100g of 17-dehydroxyacetate prednisone, 1500ml of chloroform, 15g of 20% phosphoric acid, stir to dissolve the solid completely, control the temperature to 20-25°C, and add 1500g of 3% phosphoric acid dropwise within 1-1.5 hours Potassium permanganate aqueous solution, after dripping, react at 20-25°C for 1-2 hours, TLC controls the reaction end point, after the reaction, recover 90-95% of the solvent chloroform by vacuum distillation below 35°C for the next batch Oxidation reaction, the residue was cooled to room temperature, added 1000ml of pure water for water analysis, filtered to obtain the oxide: 16a-hydroxyprednisone acetate crude product; the crude product was recrystallized with 1000ml50% alcohol aqueous solution to obtain 16a-hydroxyprednisone acetate Pine 91.6g, HPLC content 98.0%, weight yield about 91.6%;
[0044] B. Preparation of protection:
[0045] In a 1000ml three-neck flask, add...
Embodiment 3
[0052] A. Preparation of oxides:
[0053]In a 5000ml three-neck flask, add 100g of 17-dehydroxyprednisone acetate, 1500ml of DME, 50g of 20% acetic acid, stir to dissolve the solid completely, control the temperature to 20-25°C, and dropwise add 900g of 5% acetic acid within 1-1.5 hours Potassium manganate aqueous solution, after dripping, react at 20-25°C for another 1-2 hours, TLC controls the reaction end point, after the reaction, 90-95% of the solvent DME is recovered by vacuum distillation below 35°C for the next batch oxidation Reaction, the residue is cooled to room temperature, add 1000ml pure water for water analysis, filter to obtain the oxide compound: 16a-hydroxy prednisone acetate crude product; the crude product is recrystallized with 1000ml50% alcohol aqueous solution to obtain 16a-hydroxy prednisone acetate 92.5g, HPLC content 97.6%, weight yield about 92.5%;
[0054] B. Preparation of protection:
[0055] In a 1000ml three-neck flask, add 100g of the oxide ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com