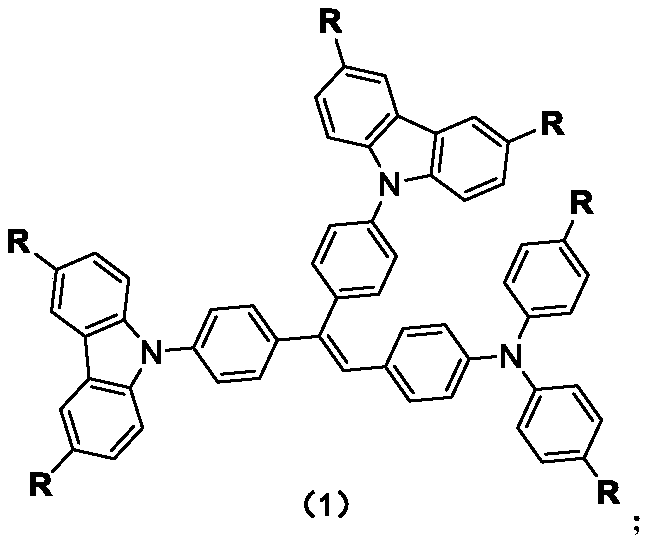
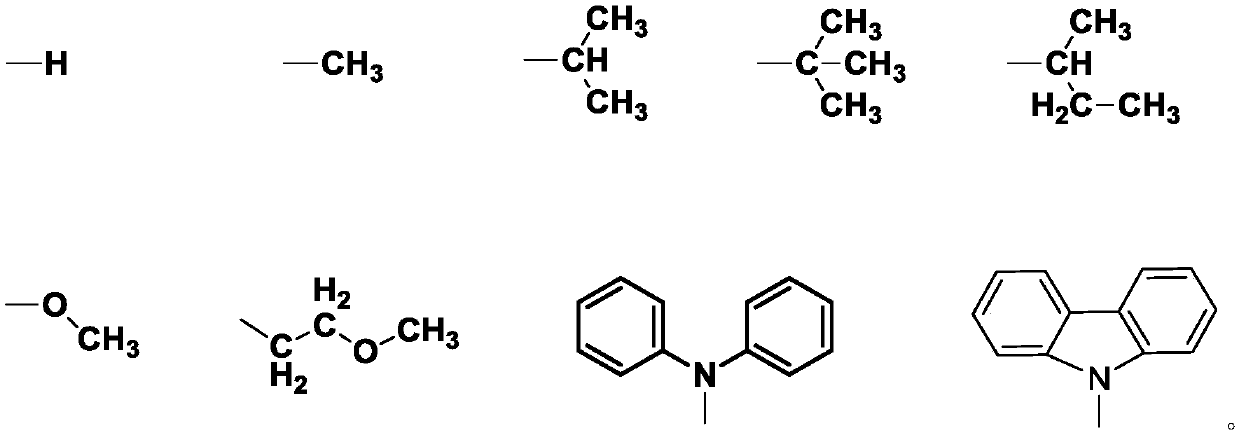
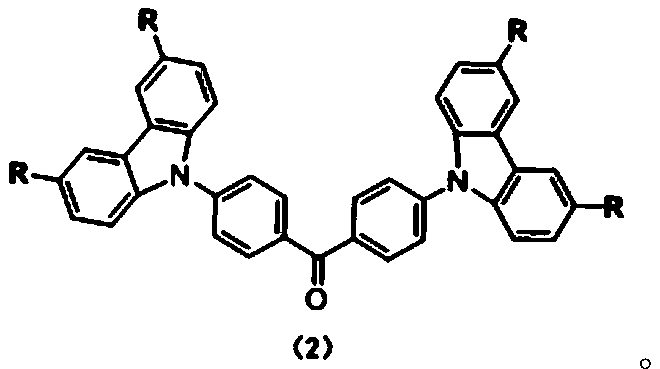
Carbazole-N-yldistyryltriphenylamine derivative hole-transport material and preparation method thereof
A technology of distyryl triphenylamine and hole transport material, which is applied in the field of carbazole-N-based distyryl triphenylamine derivative hole transport material and preparation thereof, and can solve the problem that cannot meet the practical application of optoelectronic devices , poor solubility of styrene materials, low glass transition temperature and other problems, to achieve the effect of high yield, convenient purification and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] The synthesis of example 1 methyl trianilino styrene carbazole
[0035] (1) Synthesis of Benzophenone Carbazole
[0036] N 2 Under protection, add carbazole (16.71g, 0.1mol) to a 250ml four-neck round bottom flask, add 150ml of anhydrous DMF as a solvent, then add potassium tert-butoxide (9.8g, 0.12mol), and heat to 65°C in an oil bath , stirred for 10 min. Add 4,4'-difluorobenzophenone (10.93 g, 0.5 mol) to the reactant and react at 65° C. for 12 h. Cool to room temperature, pour into ice-water mixture at 0 °C, filter, and wash three times with deionized water. Dry to give crude product. The crude product was recrystallized with acetone to obtain 24.13 g of a yellow-green solid with a yield of 94.1%.
[0037] 1 H NMR (400MHz, CDCl 3 )δ8.20(q, 2H), δ7.87–7.82(d, 1H), δ7.60(d, 1H), δ7.547.43(t, 1H), δ7.36(t, 1H)
[0038]
[0039] (2) Synthesis of Methyltrianiline Phosphate Ylide Salt
[0040] N 2 Under protection, in the 100mL four-neck flask, add methyltrip...
example 2
[0047] The synthesis of example 2 methyltriphenylamine styrene methyl carbazole
[0048] The synthesis of example 2 refers to the synthetic method of example 1, and the carbazole used is a methyl-substituted carbazole, and the color of the pure product is light yellow, and the productive rate is 71.2%.
[0049] The ratio of methylcarbazole, potassium tert-butoxide and 4,4'-difluorobenzophenone is: 3:3:1
[0050] The ratio of methylcarbazolylbenzophenone, sodium tert-butoxide and triphenylamine ylide salt is: 3:7:1
[0051]
example 3
[0052] Synthesis of Example 3 Isopropyltriphenylamineethylene Isopropylcarbazole
[0053] The synthesis of example 3 refers to the synthetic method of example 1, and the carbazole used is isopropyl substituted carbazole, and the color of pure product is pale yellow, and productive rate is 71.2%
[0054] The ratio of tert-butylcarbazole, potassium tert-butoxide and 4,4'-difluorobenzophenone is: 3:3:1
[0055] The ratio of isopropyltriphenylamine benzyl alcohol to triphenylphosphine hydrobromide is: 1:2
[0056] The ratio of tert-butylcarbazophenone, sodium tert-butoxide and triphenylamine ylide salt is: 2:6:1
[0057]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap