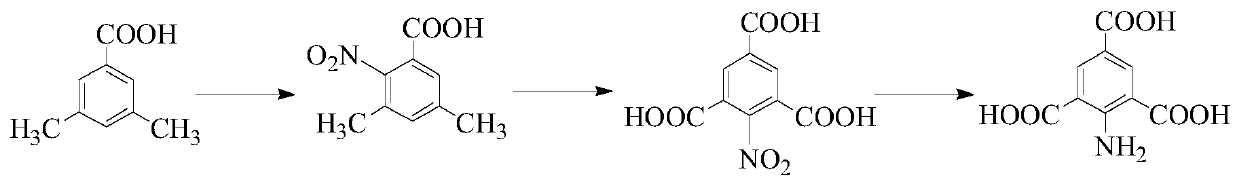
Method for synthetizing 2-amino-1, 3, 5-benzenetricarboxylic acid and application thereof for preparation of NH2-MOF-808
A technology of NH2-MOF-808 and benzenetricarboxylic acid, which is applied in the preparation of nitro compounds, organic compounds, and cyanide reaction, etc. It can solve the problems of difficult control, high price, and difficult purification, and achieve easy operation , simple preparation process, simple and effective synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
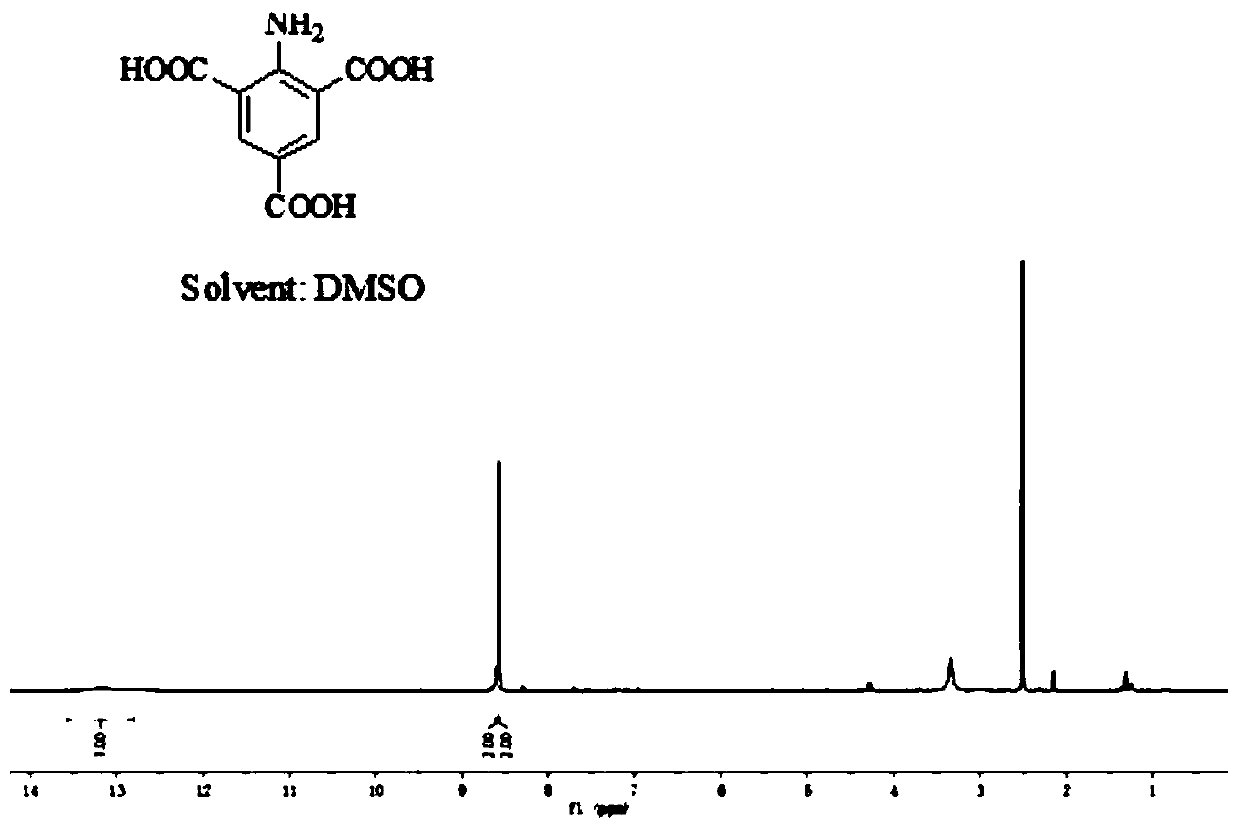
[0035] A highly efficient synthetic method for 2-amino-1,3,5-benzenetricarboxylic acid, comprising the following steps:
[0036] Step 1: Weigh 7.9g of 3,5-dimethylbenzoic acid and dissolve it in 20mL of acetic acid solution, add 8.8mL of concentrated nitric acid, and heat to 80°C. Add 7.9 mL of concentrated sulfuric acid dropwise into the mixture with a dropping funnel, and react at 80°C for 30 minutes. After the reaction, collect the solid precipitate by filtration to obtain 2-nitro-3,5-dimethylbenzoic acid. The yield is 92%.
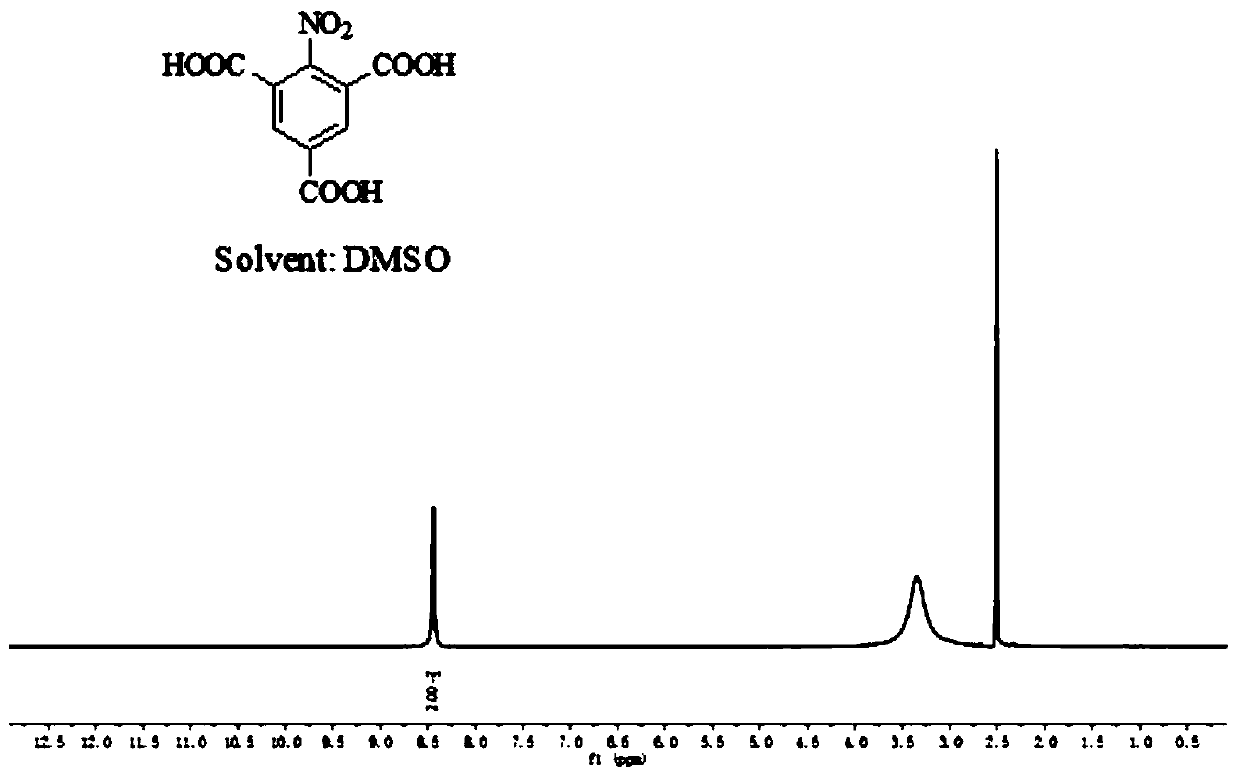
[0037] Step 2: Weigh 2g of 2-nitro-3,5-dimethylbenzoic acid obtained in step 1 and add it to 20mL of water, add 1.4g of sodium hydroxide, the pH of the test solution is alkaline, weigh 6.48g of permanganate Potassium was added three times in about 4 hours under ice bath conditions, and then reacted at 60°C for 10 hours. After the end, add ethanol to remove unreacted potassium permanganate and filter it with a sand core funnel. Take the filtrate and ad...
Embodiment 2
[0043] Efficient Synthesis of 2-Amino-1,3,5-Benzenetricarboxylic Acid and NH 2 -The preparation method of MOF-808, comprises the following steps:
[0044] Step 1: Weigh 7.9g of 3,5-dimethylbenzoic acid and dissolve it in 20mL of acetic acid solution, add 8.8mL of concentrated nitric acid, and heat to 80°C. Add 7.9 mL of concentrated sulfuric acid dropwise into the mixture with a dropping funnel, and react at 80°C for 30 minutes. After the reaction, collect the solid precipitate by filtration to obtain 2-nitro-3,5-dimethylbenzoic acid. The yield is 90%.
[0045] Step 2: Weigh 2g of 2-nitro-3,5-dimethylbenzoic acid obtained in step 1 and add it to 20mL of water, add 1.4g of sodium hydroxide, the pH of the test solution is alkaline, weigh 6.48g of permanganate Potassium was added three times in about 4 hours under ice bath conditions, and then reacted at 60°C for 10 hours. After the end, add ethanol to remove unreacted potassium permanganate and filter it with a sand core funne...
Embodiment 3
[0050] Efficient Synthesis of 2-Amino-1,3,5-Benzenetricarboxylic Acid and NH 2 -The preparation method of MOF-808, comprises the following steps:
[0051] Step 1: Weigh 7.9g of 3,5-dimethylbenzoic acid and dissolve it in 20mL of acetic acid solution, add 8.8mL of concentrated nitric acid, and heat to 80°C. Add 7.9 mL of concentrated sulfuric acid dropwise into the mixture with a dropping funnel, and react at 80°C for 30 minutes. After the reaction, collect the solid precipitate by filtration to obtain 2-nitro-3,5-dimethylbenzoic acid. The yield is 93%.
[0052] Step 2: Weigh 2g of 2-nitro-3,5-dimethylbenzoic acid obtained in step 1 and add it to 20mL of water, add 1.4g of sodium hydroxide, the pH of the test solution is alkaline, weigh 6.48g of permanganate Potassium was added three times in about 4 hours under ice bath conditions, and then reacted at 60°C for 10 hours. After the end, add ethanol to remove unreacted potassium permanganate and filter it with a sand core funne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com